Spontaneous apoptosis of cells in therapeutic stem cell preparation exert immunomodulatory effects through release of phosphatidylserine
- PMID: 34262012
- PMCID: PMC8280232
- DOI: 10.1038/s41392-021-00688-z
Spontaneous apoptosis of cells in therapeutic stem cell preparation exert immunomodulatory effects through release of phosphatidylserine
Erratum in
-
Correction: Spontaneous apoptosis of cells in therapeutic stem cell preparation exert immunomodulatory effects through release of phosphatidylserine.Signal Transduct Target Ther. 2022 Jan 13;7(1):13. doi: 10.1038/s41392-021-00862-3. Signal Transduct Target Ther. 2022. PMID: 35027536 Free PMC article. No abstract available.
Abstract
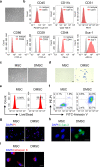
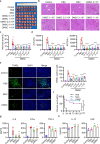
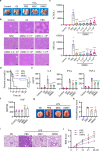
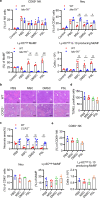
Mesenchymal stem cell (MSC)-mediated immunomodulation has been harnessed for the treatment of human diseases, but its underlying mechanism has not been fully understood. Dead cells, including apoptotic cells have immunomodulatory properties. It has been repeatedly reported that the proportion of nonviable MSCs in a MSC therapeutic preparation varied from 5~50% in the ongoing clinical trials. It is conceivable that the nonviable cells in a MSC therapeutic preparation may play a role in the therapeutic effects of MSCs. We found that the MSC therapeutic preparation in the present study had about 5% dead MSCs (DMSCs), characterized by apoptotic cells. Namely, 1 × 106 MSCs in the preparation contained about 5 × 104 DMSCs. We found that the treatment with even 5 × 104 DMSCs alone had the equal therapeutic effects as with 1 × 106 MSCs. This protective effect of the dead MSCs alone was confirmed in four mouse models, including concanavalin A (ConA)- and carbon tetrachloride (CCl4)-induced acute liver injury, LPS-induced lung injury and spinal cord injury. We also found that the infused MSCs died by apoptosis in vivo. Furthermore, the therapeutic effect was attributed to the elevated level of phosphatidylserine (PS) upon the injection of MSCs or DMSCs. The direct administration of PS liposomes (PSLs) mimic apoptotic cell fragments also exerted the protective effects as MSCs and DMSCs. The Mer tyrosine kinase (MerTK) deficiency or the knockout of chemokine receptor C-C motif chemokine receptor 2 (CCR2) reversed these protective effects of MSCs or DMSCs. These results revealed that DMSCs alone in the therapeutic stem cell preparation or the apoptotic cells induced in vivo may exert the same immunomodulatory property as the "living MSCs preparation" through releasing PS, which was further recognized by MerTK and participated in modulating immune cells.
© 2021. The Author(s).
Conflict of interest statement
All authors declare no competing interests. C.H. is the editorial board member of Signal Transduction and Targeted Therapy, but he has not been involved in the process of the manuscript handling.
Figures



Comment in
-
A new insight for stem cell therapy: apoptotic stem cells as a key player.Signal Transduct Target Ther. 2022 Aug 28;7(1):299. doi: 10.1038/s41392-022-01066-z. Signal Transduct Target Ther. 2022. PMID: 36031637 Free PMC article. No abstract available.
References
-
- Hofmann M, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–2202. - PubMed
-
- Lee KC, Lin HC, Huang YH, Hung SC. Allo-transplantation of mesenchymal stem cells attenuates hepatic injury through IL1Ra dependent macrophage switch in a mouse model of liver disease. J. Hepatol. 2015;63:1405–1412. - PubMed
-
- Lee JS, et al. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28:1099–1106. - PubMed
-
- Banas A, et al. IFATS collection: in vivo therapeutic potential of human adipose tissue mesenchymal stem cells after transplantation into mice with liver injury. Stem Cells. 2008;26:2705–2712. - PubMed
-
- Islam D, et al. Identification and modulation of microenvironment is crucial for effective mesenchymal stromal cell therapy in acute lung injury. Am. J. Respir. Crit. Care Med. 2019;199:1214–1224. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

