Novel FOXM1 inhibitor identified via gene network analysis induces autophagic FOXM1 degradation to overcome chemoresistance of human cancer cells
- PMID: 34262016
- PMCID: PMC8280155
- DOI: 10.1038/s41419-021-03978-0
Novel FOXM1 inhibitor identified via gene network analysis induces autophagic FOXM1 degradation to overcome chemoresistance of human cancer cells
Abstract
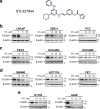
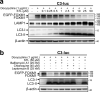
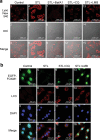
FOXM1 transcription factor is an oncogene and a master regulator of chemoresistance in multiple cancers. Pharmacological inhibition of FOXM1 is a promising approach but has proven to be challenging. We performed a network-centric transcriptomic analysis to identify a novel compound STL427944 that selectively suppresses FOXM1 by inducing the relocalization of nuclear FOXM1 protein to the cytoplasm and promoting its subsequent degradation by autophagosomes. Human cancer cells treated with STL427944 exhibit increased sensitivity to cytotoxic effects of conventional chemotherapeutic treatments (platinum-based agents, 5-fluorouracil, and taxanes). RNA-seq analysis of STL427944-induced gene expression changes revealed prominent suppression of gene signatures characteristic for FOXM1 and its downstream targets but no significant changes in other important regulatory pathways, thereby suggesting high selectivity of STL427944 toward the FOXM1 pathway. Collectively, the novel autophagy-dependent mode of FOXM1 suppression by STL427944 validates a unique pathway to overcome tumor chemoresistance and improve the efficacy of treatment with conventional cancer drugs.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
FOXM1 expression is significantly associated with chemotherapy resistance and adverse prognosis in non-serous epithelial ovarian cancer patients.J Exp Clin Cancer Res. 2017 May 8;36(1):63. doi: 10.1186/s13046-017-0536-y. J Exp Clin Cancer Res. 2017. PMID: 28482906 Free PMC article.
-
Novel FOXM1 inhibitor STL001 sensitizes human cancers to a broad-spectrum of cancer therapies.Cell Death Discov. 2024 May 2;10(1):211. doi: 10.1038/s41420-024-01929-0. Cell Death Discov. 2024. PMID: 38697979 Free PMC article.
-
MicroRNA-149 Increases the Sensitivity of Colorectal Cancer Cells to 5-Fluorouracil by Targeting Forkhead Box Transcription Factor FOXM1.Cell Physiol Biochem. 2016;39(2):617-29. doi: 10.1159/000445653. Epub 2016 Jul 15. Cell Physiol Biochem. 2016. PMID: 27415661
-
FOXM1 (Forkhead box M1) in tumorigenesis: overexpression in human cancer, implication in tumorigenesis, oncogenic functions, tumor-suppressive properties, and target of anticancer therapy.Adv Cancer Res. 2013;119:191-419. doi: 10.1016/B978-0-12-407190-2.00016-2. Adv Cancer Res. 2013. PMID: 23870513 Review.
-
Regulation of the master regulator FOXM1 in cancer.Cell Commun Signal. 2018 Sep 12;16(1):57. doi: 10.1186/s12964-018-0266-6. Cell Commun Signal. 2018. PMID: 30208972 Free PMC article. Review.
Cited by
-
FOXM1, MEK, and CDK4/6: New Targets for Malignant Peripheral Nerve Sheath Tumor Therapy.Int J Mol Sci. 2023 Sep 2;24(17):13596. doi: 10.3390/ijms241713596. Int J Mol Sci. 2023. PMID: 37686402 Free PMC article. Review.
-
Molecular classifications of prostate cancer: basis for individualized risk stratification and precision therapy.Ann Med. 2023;55(2):2279235. doi: 10.1080/07853890.2023.2279235. Epub 2023 Nov 8. Ann Med. 2023. PMID: 37939258 Free PMC article. Review.
-
Gene expression analysis during progression of malignant meningioma compared to benign meningioma.J Neurosurg. 2022 Sep 16;138(5):1302-1312. doi: 10.3171/2022.7.JNS22585. Print 2023 May 1. J Neurosurg. 2022. PMID: 36115056 Free PMC article.
-
Peptide mimetic NC114 induces growth arrest by preventing PKCδ activation and FOXM1 nuclear translocation in colorectal cancer cells.FEBS Open Bio. 2024 Apr;14(4):695-720. doi: 10.1002/2211-5463.13784. Epub 2024 Mar 1. FEBS Open Bio. 2024. PMID: 38425293 Free PMC article.
-
The Promise of Combination Therapies with FOXM1 Inhibitors for Cancer Treatment.Cancers (Basel). 2024 Feb 12;16(4):756. doi: 10.3390/cancers16040756. Cancers (Basel). 2024. PMID: 38398147 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

