CD147 receptor is essential for TFF3-mediated signaling regulating colorectal cancer progression
- PMID: 34262017
- PMCID: PMC8280106
- DOI: 10.1038/s41392-021-00677-2
CD147 receptor is essential for TFF3-mediated signaling regulating colorectal cancer progression
Abstract
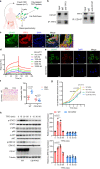
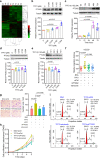
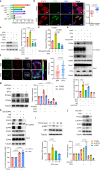
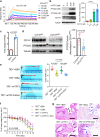
Major gaps in understanding the molecular mechanisms of colorectal cancer (CRC) progression and intestinal mucosal repair have hampered therapeutic development for gastrointestinal disorders. Trefoil factor 3 (TFF3) has been reported to be involved in CRC progression and intestinal mucosal repair; however, how TFF3 drives tumors to become more aggressive or metastatic and how TFF3 promotes intestinal mucosal repair are still poorly understood. Here, we found that the upregulated TFF3 in CRC predicted a worse overall survival rate. TFF3 deficiency impaired mucosal restitution and adenocarcinogenesis. CD147, a membrane protein, was identified as a binding partner for TFF3. Via binding to CD147, TFF3 enhanced CD147-CD44s interaction, resulting in signal transducer and activator of transcription 3 (STAT3) activation and prostaglandin G/H synthase 2 (PTGS2) expression, which were indispensable for TFF3-induced migration, proliferation, and invasion. PTGS2-derived PGE2 bound to prostaglandin E2 receptor EP4 subtype (PTGER4) and contributed to TFF3-stimulated CRC progression. Solution NMR studies of the TFF3-CD147 interaction revealed the key residues critical for TFF3 binding and the induction of PTGS2 expression. The ability of TFF3 to enhance mucosal restitution was weakened by a PTGS2 inhibitor. Blockade of TFF3-CD147 signaling using competitive inhibitory antibodies or a PTGS2 inhibitor reduced CRC lung metastasis in mice. Our findings bring strong evidence that CD147 is a novel receptor for TFF3 and PTGS2 signaling is critical for TFF3-induced mucosal restitution and CRC progression, which widens and deepens the understanding of the molecular function of trefoil factors.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

