DHA exhibits synergistic therapeutic efficacy with cisplatin to induce ferroptosis in pancreatic ductal adenocarcinoma via modulation of iron metabolism
- PMID: 34262021
- PMCID: PMC8280115
- DOI: 10.1038/s41419-021-03996-y
DHA exhibits synergistic therapeutic efficacy with cisplatin to induce ferroptosis in pancreatic ductal adenocarcinoma via modulation of iron metabolism
Abstract
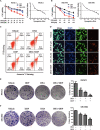
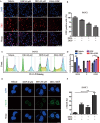
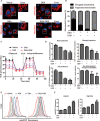
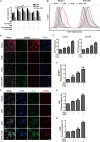
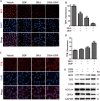
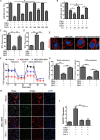
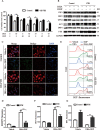
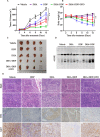
Pancreatic ductal adenocarcinoma (PDAC) is an extremely lethal cancer with limited treatment options. Cisplatin (DDP) is used as a mainstay of chemotherapeutic agents in combination with other drugs or radiotherapy for PDAC therapy. However, DDP exhibits severe side-effects that can lead to discontinuation of therapy, and the acquired drug resistance of tumor cells presents serious clinical obstacles. Therefore, it is imperative to develop a more effective and less toxic therapeutic strategy. We and others have previously discovered that dihydroartemisinin (DHA) represents a safe and promising therapeutic agent to preferentially induce cancer cell ferroptosis. In the present study, we find that DHA could intensively strengthen the cytotoxicity of DDP and significantly reduce its effective concentrations both in vitro and in vivo. Combination of DHA and DDP synergistically inhibits the proliferation and induces DNA damage of PDAC cells. Mechanically, the combinative treatment impairs mitochondrial homeostasis, characterized by destroyed mitochondrial morphology, decreased respiratory capacity, reduced ATP production, and accumulated mitochondria-derived ROS. Further studies show that ferroptosis contributes to the cytotoxic effects in PDAC cells under the challenge of DHA and DDP, together with catastrophic accumulation of free iron and unrestricted lipid peroxidation. Moreover, pharmacologic depleting of the free iron reservoir or reconstituted expression of FTH contributes to the tolerance of DHA/DDP-induced ferroptosis, while iron addition accelerates the ferroptotic cell death. In summary, these results provide experimental evidence that DHA acts synergistically with DDP and renders PDAC cells vulnerable to ferroptosis, which may act as a promising therapeutic strategy.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

