The HIF target MAFF promotes tumor invasion and metastasis through IL11 and STAT3 signaling
- PMID: 34262028
- PMCID: PMC8280233
- DOI: 10.1038/s41467-021-24631-6
The HIF target MAFF promotes tumor invasion and metastasis through IL11 and STAT3 signaling
Abstract
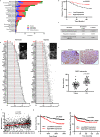
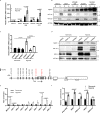
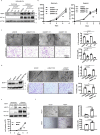
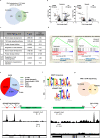
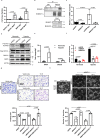
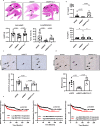
Hypoxia plays a critical role in tumor progression including invasion and metastasis. To determine critical genes regulated by hypoxia that promote invasion and metastasis, we screen fifty hypoxia inducible genes for their effects on invasion. In this study, we identify v-maf musculoaponeurotic fibrosarcoma oncogene homolog F (MAFF) as a potent regulator of tumor invasion without affecting cell viability. MAFF expression is elevated in metastatic breast cancer patients and is specifically correlated with hypoxic tumors. Combined ChIP- and RNA-sequencing identifies IL11 as a direct transcriptional target of the heterodimer between MAFF and BACH1, which leads to activation of STAT3 signaling. Inhibition of IL11 results in similar levels of metastatic suppression as inhibition of MAFF. This study demonstrates the oncogenic role of MAFF as an activator of the IL11/STAT3 pathways in breast cancer.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–1416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

