Intracellular lipid droplet accumulation occurs early following viral infection and is required for an efficient interferon response
- PMID: 34262037
- PMCID: PMC8280141
- DOI: 10.1038/s41467-021-24632-5
Intracellular lipid droplet accumulation occurs early following viral infection and is required for an efficient interferon response
Abstract
Lipid droplets (LDs) are increasingly recognized as critical organelles in signalling events, transient protein sequestration and inter-organelle interactions. However, the role LDs play in antiviral innate immune pathways remains unknown. Here we demonstrate that induction of LDs occurs as early as 2 h post-viral infection, is transient and returns to basal levels by 72 h. This phenomenon occurs following viral infections, both in vitro and in vivo. Virally driven in vitro LD induction is type-I interferon (IFN) independent, and dependent on Epidermal Growth Factor Receptor (EGFR) engagement, offering an alternate mechanism of LD induction in comparison to our traditional understanding of their biogenesis. Additionally, LD induction corresponds with enhanced cellular type-I and -III IFN production in infected cells, with enhanced LD accumulation decreasing viral replication of both Herpes Simplex virus 1 (HSV-1) and Zika virus (ZIKV). Here, we demonstrate, that LDs play vital roles in facilitating the magnitude of the early antiviral immune response specifically through the enhanced modulation of IFN following viral infection, and control of viral replication. By identifying LDs as a critical signalling organelle, this data represents a paradigm shift in our understanding of the molecular mechanisms which coordinate an effective antiviral response.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
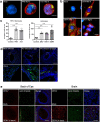
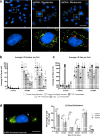


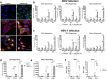


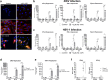

Comment in
-
Host upregulation of lipid droplets drives antiviral responses.Cell Stress. 2021 Aug 25;5(9):143-145. doi: 10.15698/cst2021.09.256. eCollection 2021 Sep. Cell Stress. 2021. PMID: 34527863 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

