Deep carbon cycle constrained by carbonate solubility
- PMID: 34262043
- PMCID: PMC8280166
- DOI: 10.1038/s41467-021-24533-7
Deep carbon cycle constrained by carbonate solubility
Abstract
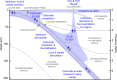
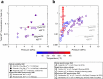
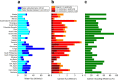
Earth's deep carbon cycle affects atmospheric CO2, climate, and habitability. Owing to the extreme solubility of CaCO3, aqueous fluids released from the subducting slab could extract all carbon from the slab. However, recycling efficiency is estimated at only around 40%. Data from carbonate inclusions, petrology, and Mg isotope systematics indicate Ca2+ in carbonates is replaced by Mg2+ and other cations during subduction. Here we determined the solubility of dolomite [CaMg(CO3)2] and rhodochrosite (MnCO3), and put an upper limit on that of magnesite (MgCO3) under subduction zone conditions. Solubility decreases at least two orders of magnitude as carbonates become Mg-rich. This decreased solubility, coupled with heterogeneity of carbon and water subduction, may explain discrepancies in carbon recycling estimates. Over a range of slab settings, we find aqueous dissolution responsible for mobilizing 10 to 92% of slab carbon. Globally, aqueous fluids mobilise [Formula: see text]% ([Formula: see text] Mt/yr) of subducted carbon from subducting slabs.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Galvez, M. E. & Pubellier, M. How do subduction zones regulate the carbon cycle? in DeepCarbon: Past to Present, 276–312 (Cambridge University Press, 2019).
-
- Van Keken, P. E., Hacker, B. R., Syracuse, E. M. & Abers, G. A. Subduction factory: 4. Depth-dependent flux of H2O from subducting slabs worldwide. J. Geophys. Res. Solid Earth116, B01401 (2011).
-
- Clift PD. A revised budget for Cenozoic sedimentary carbon subduction. Rev. Geophys. 2017;55:97–125. doi: 10.1002/2016RG000531. - DOI
-
- Li K, Li L, Pearson DG, Stachel T. Diamond isotope compositions indicate altered igneous oceanic crust dominates deep carbon recycling. Earth Planet. Sci. Lett. 2019;516:190–201. doi: 10.1016/j.epsl.2019.03.041. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

