The Development of Disease-Modifying Therapies for Osteoarthritis (DMOADs): The Evidence to Date
- PMID: 34262259
- PMCID: PMC8273751
- DOI: 10.2147/DDDT.S295224
The Development of Disease-Modifying Therapies for Osteoarthritis (DMOADs): The Evidence to Date
Abstract
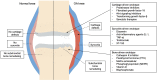
Osteoarthritis (OA) is a complex heterogeneous articular disease with multiple joint tissue involvement of varying severity and no regulatory-agency-approved disease-modifying drugs (DMOADs). In this review, we discuss the reasons necessitating the development of DMOADs for OA management, the classifications of clinical phenotypes or molecular/mechanistic endotypes from the viewpoint of targeted drug discovery, and then summarize the efficacy and safety profile of a range of targeted drugs in Phase 2 and 3 clinical trials directed to cartilage-driven, bone-driven, and inflammation-driven endotypes. Finally, we briefly put forward the reasons for failures in OA clinical trials and possible steps to overcome these barriers.
Keywords: DMOADs; disease-modifying drugs; endotype; intra-articular therapy; osteoarthritis; phenotype.
© 2021 Oo et al.
Conflict of interest statement
DJH provides consulting advice on scientific advisory boards for Pfizer, Lilly, TLCBio, Novartis, Tissuegene, Biobone. CL has provided consulting advice for Merck Serono and Galapagos Pharmaceuticals, and receives research funding from numerous pharmaceutical companies (Fidia Farmaceutici, Inter-K Peptide Therapeutics Ltd, Taisho Pharmaceutical Co. Ltd, Concentric Analgesics Inc, Cynata Therapeutics, CEVA Animal Health, Regeneus) through specific services/testing contract research agreements between and managed by The University of Sydney or the NSLHD. The authors report no other conflicts of interest in this work.
Figures
References
-
- Hawker GA. Osteoarthritis is a serious disease. Clin Exp Rheumatol. 2019;37 Suppl 120(5):3–6. - PubMed
-
- OARSI TP-cCfOPo. OARSI White Paper- OA as a Serious Disease; 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical