Mapping T Cell Responses to Native and Neo-Islet Antigen Epitopes in at Risk and Type 1 Diabetes Subjects
- PMID: 34262563
- PMCID: PMC8274489
- DOI: 10.3389/fimmu.2021.675746
Mapping T Cell Responses to Native and Neo-Islet Antigen Epitopes in at Risk and Type 1 Diabetes Subjects
Abstract
Aims: Recent studies highlight the potentially important role of neoepitopes in breaking immune tolerance in type 1 diabetes. T cell reactivity to these neoepitopes has been reported, but how this response compares quantitatively and phenotypically with previous reports on native epitopes is not known. Thus, an understanding of the relationship between native and neoepitopes and their role as tolerance breakers or disease drivers in type 1 diabetes is required. We set out to compare T cell reactivity and phenotype against a panel of neo- and native islet autoantigenic epitopes to examine how this relates to stages of type 1 diabetes development.
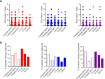
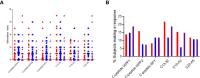
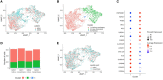
Methods: Fifty-four subjects comprising patients with T1D, and autoantibody-positive unaffected family members were tested against a panel of neo- and native epitopes by ELISPOT (IFN-γ, IL-10, and IL-17). A further subset of two patients was analyzed by Single Cell Immune Profiling (RNAseq and TCR α/β) after stimulation with pools of native and neoepitope peptides.
Results: T cell responses to native and neoepitopes were present in patients with type 1 diabetes and at-risk subjects, and overall, there were no significant differences in the frequency, magnitude, or phenotype between the two sets of peptide stimuli. Single cell RNAseq on responder T cells revealed a similar profile in T1D patients stimulated with either neo- or native epitopes. A pro-inflammatory gene expression profile (TNF-α, IFN-γ) was dominant in both native and neoepitope stimulated T cells. TCRs with identical clonotypes were found in T cell responding to both native and neoepitopes.
Conclusion/interpretation: These data suggest that in peripheral blood, T cell responses to both native and neoepitopes are similar in terms of frequency and phenotype in patients with type 1 diabetes and high-risk unaffected family members. Furthermore, using a combination of transcriptomic and clonotypic analyses, albeit using a limited panel of peptides, we show that neoepitopes are comparable to native epitopes currently in use for immune-monitoring studies.
Keywords: T cell receptor; T cells; autoantibody; cytokines; genes; neoepitopes; transcriptome; type 1 diabetes.
Copyright © 2021 Arif, Pujol-Autonell, Kamra, Williams, Yusuf, Domingo-Vila, Shahrabi, Pollock, Khatri, Peakman, Tree and Lorenc.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

