Differential Cytokine Responses in Hospitalized COVID-19 Patients Limit Efficacy of Remdesivir
- PMID: 34262564
- PMCID: PMC8275132
- DOI: 10.3389/fimmu.2021.680188
Differential Cytokine Responses in Hospitalized COVID-19 Patients Limit Efficacy of Remdesivir
Abstract
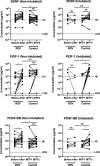
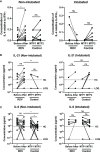
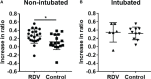
A significant proportion of COVID-19 patients will progress to critical illness requiring invasive mechanical ventilation. This accentuates the need for a therapy that can reduce the severity of COVID-19. Clinical trials have shown the effectiveness of remdesivir in shortening recovery time and decreasing progression to respiratory failure and mechanical ventilation. However, some studies have highlighted its lack of efficacy in patients on high-flow oxygen and mechanical ventilation. This study uncovers some underlying immune response differences between responders and non-responders to remdesivir treatment. Immunological analyses revealed an upregulation of tissue repair factors BDNF, PDGF-BB and PIGF-1, as well as an increase in ratio of Th2-associated cytokine IL-4 to Th1-associated cytokine IFN-γ. Serological profiling of IgG subclasses corroborated this observation, with significantly higher magnitude of increase in Th2-associated IgG2 and IgG4 responses. These findings help to identify the mechanisms of immune regulation accompanying successful remdesivir treatment in severe COVID-19 patients.
Keywords: COVID-19; T-cells; disease progression; remdesivir (GS-5734); tissue repair.
Copyright © 2021 Chan, Young, Fong, Ding, Goh, Chee, Tan, Kalimuddin, Tambyah, Leo, Ng, Lye and Renia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

