Epigenetic reprogramming enhances the therapeutic efficacy of osteoblast-derived extracellular vesicles to promote human bone marrow stem cell osteogenic differentiation
- PMID: 34262674
- PMCID: PMC8263905
- DOI: 10.1002/jev2.12118
Epigenetic reprogramming enhances the therapeutic efficacy of osteoblast-derived extracellular vesicles to promote human bone marrow stem cell osteogenic differentiation
Abstract
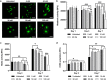
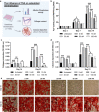
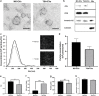
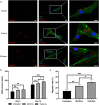
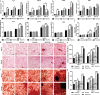
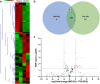
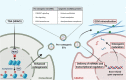
Extracellular vesicles (EVs) are emerging in tissue engineering as promising acellular tools, circumventing many of the limitations associated with cell-based therapies. Epigenetic regulation through histone deacetylase (HDAC) inhibition has been shown to increase differentiation capacity. Therefore, this study aimed to investigate the potential of augmenting osteoblast epigenetic functionality using the HDAC inhibitor Trichostatin A (TSA) to enhance the therapeutic efficacy of osteoblast-derived EVs for bone regeneration. TSA was found to substantially alter osteoblast epigenetic function through reduced HDAC activity and increased histone acetylation. Treatment with TSA also significantly enhanced osteoblast alkaline phosphatase activity (1.35-fold), collagen production (2.8-fold) and calcium deposition (1.55-fold) during osteogenic culture (P ≤ 0.001). EVs derived from TSA-treated osteoblasts (TSA-EVs) exhibited reduced particle size (1-05-fold) (P > 0.05), concentration (1.4-fold) (P > 0.05) and protein content (1.16-fold) (P ≤ 0.001) when compared to untreated EVs. TSA-EVs significantly enhanced the proliferation (1.13-fold) and migration (1.3-fold) of human bone marrow stem cells (hBMSCs) when compared to untreated EVs (P ≤ 0.05). Moreover, TSA-EVs upregulated hBMSCs osteoblast-related gene and protein expression (ALP, Col1a, BSP1 and OCN) when compared to cells cultured with untreated EVs. Importantly, TSA-EVs elicited a time-dose dependent increase in hBMSCs extracellular matrix mineralisation. MicroRNA profiling revealed a set of differentially expressed microRNAs from TSA-EVs, which were osteogenic-related. Target prediction demonstrated these microRNAs were involved in regulating pathways such as 'endocytosis' and 'Wnt signalling pathway'. Moreover, proteomics analysis identified the enrichment of proteins involved in transcriptional regulation within TSA-EVs. Taken together, our findings suggest that altering osteoblasts' epigenome accelerates their mineralisation and promotes the osteoinductive potency of secreted EVs partly due to the delivery of pro-osteogenic microRNAs and transcriptional regulating proteins. As such, for the first time we demonstrate the potential to harness epigenetic regulation as a novel engineering approach to enhance EVs therapeutic efficacy for bone repair.
Keywords: bone; epigenetics; extracellular vesicles; histone deacetylase; microRNAs; tissue engineering; trichostatin A.
© 2021 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Aasland, R. (1995). The phd finger: Implications for chromatin‐mediated transcriptional regulation. Trends in Biochemical Sciences, 20(2), 56–59. - PubMed
-
- Barbaric, I. , Perry, M. J. , Dear, T. N. , Rodrigues Da Costa, A. , Salopek, D. , Marusic, A. , Hough, T. , Wells, S. , Hunter, A. J. , Cheeseman, M. , & Brown, S. D. M. (2008). An enu‐induced mutation in the ankrd11 gene results in an osteopenia‐like phenotype in the mouse mutant yoda. Physiological Genomics, 32(3), 311–321. - PubMed
-
- Baroli, B. (2009). From natural bone grafts to tissue engineering therapeutics: Brainstorming on pharmaceutical formulative requirements and challenges. Journal of Pharmaceutical Sciences, 98(4), 1317–1375. - PubMed
-
- Bhushan, R. , Grunhagen, J. , Becker, J. , Robinson, P. N. , Ott, C. E. , & Knaus, P. (2013). Mir‐181a promotes osteoblastic differentiation through repression of tgf‐beta signaling molecules. International Journal of Biochemistry & Cell Biology, 45(3), 696–705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

