Clinical, Radiometabolic and Immunologic Effects of Olaparib in Locally Advanced Triple Negative Breast Cancer: The OLTRE Window of Opportunity Trial
- PMID: 34262869
- PMCID: PMC8273330
- DOI: 10.3389/fonc.2021.686776
Clinical, Radiometabolic and Immunologic Effects of Olaparib in Locally Advanced Triple Negative Breast Cancer: The OLTRE Window of Opportunity Trial
Abstract
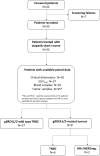
Introduction: Olaparib is effective in metastatic triple negative breast cancer (TNBC) carrying germline mutations in DNA damage repair (DDR) genes BRCA1/2 (gBRCA-mut). The OLTRE window-of-opportunity trial preliminarily investigated potential pathologic, radiometabolic and immune biomarkers of early-response to olaparib in gBRCA-wild-type (wt) TNBC and, as proof-of-concept in gBRCA-mut HER2-negative BC.
Methods: Patients received olaparib for 3 weeks (3w) before standard neoadjuvant chemotherapy and underwent multiple FDG18-PET/CT scan (basal, after olaparib), clinical assessments (basal, every 3w), tumor biopsies and blood samplings (baseline, after olaparib). Clinical and radiometabolic responses were evaluated according to RECIST1.1 and PERCIST criteria.
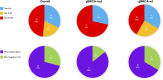
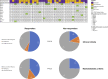
Results: 27 patients with gBRCA-wt TNBC and 8 with gBRCA-mut BC (6 TNBC, 2 HR+/HER2-negative) were enrolled. Three (11.1%) patients showed mutations in non-BRCA1/2 DDR genes and 4 (14.8%) in other genes. 3w olaparib induced 16/35 and 15/27 partial clinical and radiometabolic responses, including in 40.7% and 50.0% gBRCA-wt patients. gBRCA-mut tumors presented numerically higher tumor-infiltrating lymphocytes (TILs) levels and PD-L1 positive tumors. Clinical responders experienced a reduction in T-regs/T-eff ratio (p=0.05), B and NK lymphocytes (p=0.003 both), with an average increase in T-helpers rate (p<0.001) and CD4/CD8 ratio (p=0.02). Ki67% and TILs did not vary significantly (p=0.67 and p=0.77). A numerical increase in PD-L1 positive cases after olaparib was observed, though non-significant (p=0.134). No differences were observed according to gBRCA status and type of response.
Conclusions: Early-stage TNBC might be a target population for olaparib, irrespective of gBRCA mutations. Future trials should combine TILs, PD-L1 and gBRCA status to better identify candidates for escalated/de-escalated treatment strategies including olaparib.
Keywords: BRCA; PD-L1; TILs; homologous recombination deficiency; neoadjuvant; olaparib (Lynparza™); triple negative breast cancer; window of opportunity clinical trial.
Copyright © 2021 Schettini, Corona, Giudici, Strina, Sirico, Bernocchi, Milani, Ziglioli, Aguggini, Azzini, Barbieri, Cervoni, Cappelletti, Molteni, Lazzari, Ferrero, Ungari, Marasco, Bruson, Xumerle, Zago, Cerra, Loddo, Williams, Paris, Scambia and Generali.
Conflict of interest statement
EM, AB, LX, EZ and DC were employed by Personal Genomics Ltd. GW and ML are employed at Oncologica UK Ltd., which has received project funding from AstraZeneca outside of the submitted work. DG has declared consulting fees from Novartis, Lilly and Pfizer, research funding from LILT, Novartis, Astra-Zeneca and University of Trieste outside of the submitted work. IP has declared consulting fees from Roche, Novartis, Lilly, Pfizer, Astra-Zeneca, Pierre Fabre and Ipsen outside of the submitted work. GS has declared Grant/Research Support from MSD Italia S.r.l., consulting role for TESARO Bio Italy S.r.l. Johnson & Johnson and Clovis Oncology Italy S.r.l., outside of the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

