Insights Into Dendritic Cells in Cancer Immunotherapy: From Bench to Clinical Applications
- PMID: 34262904
- PMCID: PMC8273339
- DOI: 10.3389/fcell.2021.686544
Insights Into Dendritic Cells in Cancer Immunotherapy: From Bench to Clinical Applications
Abstract
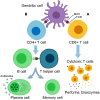
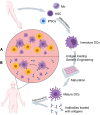
Dendritic cells (DCs) are efficient antigen-presenting cells (APCs) and potent activators of naïve T cells. Therefore, they act as a connective ring between innate and adaptive immunity. DC subsets are heterogeneous in their ontogeny and functions. They have proven to potentially take up and process tumor-associated antigens (TAAs). In this regard, researchers have developed strategies such as genetically engineered or TAA-pulsed DC vaccines; these manipulated DCs have shown significant outcomes in clinical and preclinical models. Here, we review DC classification and address how DCs are skewed into an immunosuppressive phenotype in cancer patients. Additionally, we present the advancements in DCs as a platform for cancer immunotherapy, emphasizing the technologies used for in vivo targeting of endogenous DCs, ex vivo generated vaccines from peripheral blood monocytes, and induced pluripotent stem cell-derived DCs (iPSC-DCs) to boost antitumoral immunity.
Keywords: cancer immunotherapy; cancer vaccines; dendritic cells; iPSC-DCs; induced pluripotent stem cells.
Copyright © 2021 Salah, Wang, Li, Ji, Ou, Qi and Wu.
Conflict of interest statement
HW and NQ were employed by the company Hangzhou Biaomo Biosciences Co., Ltd., Hangzhou, China and Asia Stem Cell Therapies Co., Limited, Shanghai, China. MJ was employed by the company Hangzhou Biaomo Biosciences Co., Ltd., Hangzhou, China. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Genetically modified dendritic cells in cancer therapy: implications for transfusion medicine.Transfus Med Rev. 2001 Oct;15(4):292-304. doi: 10.1053/tmrv.2001.26960. Transfus Med Rev. 2001. PMID: 11668436 Review.
-
Eliciting cytotoxic T lymphocytes against human laryngeal cancer-derived antigens: evaluation of dendritic cells pulsed with a heat-treated tumor lysate and other antigen-loading strategies for dendritic-cell-based vaccination.J Exp Clin Cancer Res. 2016 Jan 22;35:18. doi: 10.1186/s13046-016-0295-1. J Exp Clin Cancer Res. 2016. PMID: 26795730 Free PMC article.
-
The dendritic cell tool for oral cancer treatment.J Oral Maxillofac Pathol. 2019 Sep-Dec;23(3):326-329. doi: 10.4103/jomfp.JOMFP_325_19. J Oral Maxillofac Pathol. 2019. PMID: 31942108 Free PMC article.
-
Dendritic cells as orchestrators of anticancer immunity and immunotherapy.Nat Rev Clin Oncol. 2024 Apr;21(4):257-277. doi: 10.1038/s41571-024-00859-1. Epub 2024 Feb 7. Nat Rev Clin Oncol. 2024. PMID: 38326563 Review.
-
Effective induction of naive and recall T-cell responses by targeting antigen to human dendritic cells via a humanized anti-DC-SIGN antibody.Blood. 2005 Aug 15;106(4):1278-85. doi: 10.1182/blood-2005-01-0318. Epub 2005 May 5. Blood. 2005. PMID: 15878980
Cited by
-
Messenger RNA vaccines for cancer immunotherapy: progress promotes promise.J Clin Invest. 2022 Mar 15;132(6):e156211. doi: 10.1172/JCI156211. J Clin Invest. 2022. PMID: 35289317 Free PMC article. Review.
-
Heterogeneity of adherent and non‑adherent JAWS II dendritic cells.Oncol Lett. 2023 Jan 25;25(3):93. doi: 10.3892/ol.2023.13680. eCollection 2023 Mar. Oncol Lett. 2023. PMID: 36817038 Free PMC article.
-
Dynamic interactome of the MHC I peptide loading complex in human dendritic cells.Proc Natl Acad Sci U S A. 2023 Jun 20;120(25):e2219790120. doi: 10.1073/pnas.2219790120. Epub 2023 Jun 12. Proc Natl Acad Sci U S A. 2023. PMID: 37307450 Free PMC article.
-
The safety and anti-tumor effect of multiple peptides-pulsed dendritic cells combined with induced specific cytotoxic T lymphocytes for patients with solid tumors.Front Immunol. 2023 Oct 24;14:1284334. doi: 10.3389/fimmu.2023.1284334. eCollection 2023. Front Immunol. 2023. PMID: 37942324 Free PMC article.
-
Advancing Immunotherapies for HPV-Related Cancers: Exploring Novel Vaccine Strategies and the Influence of Tumor Microenvironment.Vaccines (Basel). 2023 Aug 11;11(8):1354. doi: 10.3390/vaccines11081354. Vaccines (Basel). 2023. PMID: 37631922 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

