In vitro characterization of CT-001-a short-acting factor VIIa with enhanced prohemostatic activity
- PMID: 34263099
- PMCID: PMC8265787
- DOI: 10.1002/rth2.12530
In vitro characterization of CT-001-a short-acting factor VIIa with enhanced prohemostatic activity
Abstract
Background: Traumatic injury and the associated acute bleeding are leading causes of death in people aged 1 to 44 years. Acute bleeding in pathological and surgical settings also represents a significant burden to the society. Yet there are no approved hemostatic drugs currently available. While clinically proven as an effective pro-coagulant, activated factor VII (FVIIa) use in acute bleeding has been hampered by unwanted thromboembolic events. Enhancing the ability of FVIIa to quickly stop a bleed and clear rapidly from circulation may yield an ideal molecule suitable for use in patients with acute bleeding.
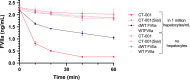
Objectives: To address this need and the current liability of FVIIa, we produced a novel FVIIa molecule (CT-001) with enhanced potency and shortened plasma residence time by cell line engineering and FVIIa protein engineering for superior efficacy for acute bleeding and safety.
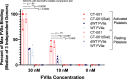
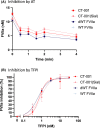
Methods: To address safety, CT-001, a FVIIa protein with 4 desialylated N-glycans was generated to promote active recognition and clearance via the asialoglycoprotein receptor. To enhance potency, the gamma-carboxylated domain was modified with P10Q and K32E, which enhanced membrane binding.
Results: Together, these changes significantly enhanced potency and clearance while retaining the ability to interact with the key hemostatic checkpoint proteins antithrombin and tissue factor pathway inhibitor.
Conclusions: These results demonstrate that a FVIIa molecule engineered to combine supra-physiological activity and shorter duration of action has the potential to overcome the current limitations of recombinant FVIIa to be a safe and effective approach to the treatment of acute bleeding.
Keywords: blood coagulation; factor VIIa; hemorrhage; hemostasis; protein engineering.
© 2021 Coagulant Therapeutics. Research and Practice in Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis (ISTH).
Figures



References
-
- Mayer SA, Brun NC, Begtrup K et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. New Engl J Med. 2008;358:2127‐2137. - PubMed
-
- Mayer SA, Brun NC, Begtrup K et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777‐785. - PubMed
-
- Hauser CJ, Boffard K, Dutton R et al. Results of the CONTROL Trial: efficacy and safety of recombinant activated factor VII in the management of refractory traumatic hemorrhage. J Trauma. 2010;69:489‐500. - PubMed
-
- Dutton RP, Parr M, Tortella BJ et al. Recombinant activated factor VII safety in trauma patients: results from the CONTROL trial. J Trauma. 2011;71:12‐19. - PubMed
LinkOut - more resources
Full Text Sources

