Biocompatibility, uptake and subcellular localization of bacterial magnetosomes in mammalian cells
- PMID: 34263139
- PMCID: PMC8243654
- DOI: 10.1039/d0na01086c
Biocompatibility, uptake and subcellular localization of bacterial magnetosomes in mammalian cells
Abstract
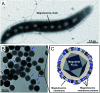
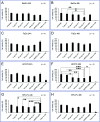
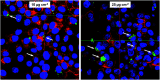
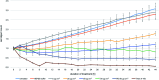
Magnetosomes represent biogenic, magnetic nanoparticles biosynthesized by magnetotactic bacteria. Subtle biological control on each step of biomineralization generates core-shell nanoparticles of high crystallinity, strong magnetization and uniform shape and size. These features make magnetosomes a promising alternative to chemically synthesized nanoparticles for many applications in the biotechnological and biomedical field, such as their usage as biosensors in medical diagnostics, as drug-delivery agents, or as contrast agents for magnetic imaging techniques. Thereby, the particles are directly applied to mammalian cells or even injected into the body. In the present work, we provide a comprehensive characterization of isolated magnetosomes as potential cytotoxic effects and particle uptake have not been well studied so far. Different cell lines including cancer cells and primary cells are incubated with increasing particle amounts, and effects on cell viability are investigated. Obtained data suggest a concentration-dependent biocompatibility of isolated magnetosomes for all tested cell lines. Furthermore, magnetosome accumulation in endolysosomal structures around the nuclei is observed. Proliferation rates are affected in the presence of increasing particle amounts; however, viability is not affected and doubling times can be restored by reducing the magnetosome concentration. In addition, we evidence magnetosome-cell interactions that are strong enough to allow for magnetic cell sorting. Overall, our study not only assesses the biocompatibility of isolated magnetosomes, but also evaluates effects on cell proliferation and the fate of internalized magnetosomes, thereby providing prerequisites for their future in vivo application as biomedical agents.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
LinkOut - more resources
Full Text Sources

