Crossing the blood-brain barrier with carbon dots: uptake mechanism and in vivo cargo delivery
- PMID: 34263140
- PMCID: PMC8243484
- DOI: 10.1039/d1na00145k
Crossing the blood-brain barrier with carbon dots: uptake mechanism and in vivo cargo delivery
Abstract
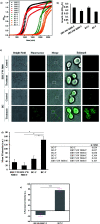
The blood-brain barrier (BBB) is a major obstacle for drug delivery to the central nervous system (CNS) such that most therapeutics lack efficacy against brain tumors or neurological disorders due to their inability to cross the BBB. Therefore, developing new drug delivery platforms to facilitate drug transport to the CNS and understanding their mechanism of transport are crucial for the efficacy of therapeutics. Here, we report (i) carbon dots prepared from glucose and conjugated to fluorescein (GluCD-F) cross the BBB in zebrafish and rats without the need of an additional targeting ligand and (ii) uptake mechanism of GluCDs is glucose transporter-dependent in budding yeast. Glucose transporter-negative strain of yeast showed undetectable GluCD accumulation unlike the glucose transporter-positive yeast, suggesting glucose-transporter-dependent GluCD uptake. We tested GluCDs' ability to cross the BBB using both zebrafish and rat models. Following the injection to the heart, wild-type zebrafish showed GluCD-F accumulation in the central canal consistent with the transport of GluCD-F across the BBB. In rats, following intravenous administration, GluCD-F was observed in the CNS. GluCD-F was localized in the gray matter (e.g. ventral horn, dorsal horn, and middle grey) of the cervical spinal cord consistent with neuronal accumulation. Therefore, neuron targeting GluCDs hold tremendous potential as a drug delivery platform in neurodegenerative disease, traumatic injury, and malignancies of the CNS.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
Authors declare no conflict of interest.
Figures




Similar articles
-
Folic Acid-Conjugated Brush Polymers Show Enhanced Blood-Brain Barrier Crossing in Static and Dynamic In Vitro Models Toward Brain Cancer Targeting Therapy.ACS Biomater Sci Eng. 2024 May 13;10(5):2894-2910. doi: 10.1021/acsbiomaterials.3c01650. Epub 2024 Mar 31. ACS Biomater Sci Eng. 2024. PMID: 38556768
-
Crossing the blood-brain-barrier with transferrin conjugated carbon dots: A zebrafish model study.Colloids Surf B Biointerfaces. 2016 Sep 1;145:251-256. doi: 10.1016/j.colsurfb.2016.05.007. Epub 2016 May 5. Colloids Surf B Biointerfaces. 2016. PMID: 27187189
-
Lipid nanoparticles strategies to modify pharmacokinetics of central nervous system targeting drugs: Crossing or circumventing the blood-brain barrier (BBB) to manage neurological disorders.Adv Drug Deliv Rev. 2022 Oct;189:114485. doi: 10.1016/j.addr.2022.114485. Epub 2022 Aug 12. Adv Drug Deliv Rev. 2022. PMID: 35970274 Review.
-
Aspect Ratio of PEGylated Upconversion Nanocrystals Affects the Cellular Uptake In Vitro and In Vivo.Acta Biomater. 2022 Jul 15;147:403-413. doi: 10.1016/j.actbio.2022.05.029. Epub 2022 May 21. Acta Biomater. 2022. PMID: 35605956
-
Cellular and Molecular Targeted Drug Delivery in Central Nervous System Cancers: Advances in Targeting Strategies.Curr Top Med Chem. 2020;20(30):2762-2776. doi: 10.2174/1568026620666200826122402. Curr Top Med Chem. 2020. PMID: 32851962 Review.
Cited by
-
Dopamine- and Grape-Seed-Extract-Loaded Solid Lipid Nanoparticles: Interaction Studies between Particles and Differentiated SH-SY5Y Neuronal Cell Model of Parkinson's Disease.Molecules. 2024 Apr 13;29(8):1774. doi: 10.3390/molecules29081774. Molecules. 2024. PMID: 38675592 Free PMC article.
-
Anticancer activity of quantum size carbon dots: opportunities and challenges.Discov Nano. 2024 Aug 5;19(1):122. doi: 10.1186/s11671-024-04069-7. Discov Nano. 2024. PMID: 39103694 Free PMC article. Review.
-
Breaking the barrier: Nanoparticle-enhanced radiotherapy as the new vanguard in brain tumor treatment.Front Pharmacol. 2024 Jul 3;15:1394816. doi: 10.3389/fphar.2024.1394816. eCollection 2024. Front Pharmacol. 2024. PMID: 39021831 Free PMC article. Review.
-
A Historical Review of Brain Drug Delivery.Pharmaceutics. 2022 Jun 16;14(6):1283. doi: 10.3390/pharmaceutics14061283. Pharmaceutics. 2022. PMID: 35745855 Free PMC article. Review.
-
Recent Advances in Carbon Dots for In Vitro/Vivo Fluorescent Bioimaging: A Mini-Review.Front Chem. 2022 May 5;10:905475. doi: 10.3389/fchem.2022.905475. eCollection 2022. Front Chem. 2022. PMID: 35601546 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

