Sphingosine-1-phosphate Receptor-1 Agonist Averts the De Novo Generation of Autoreactive T-cells in Murine Acute Graft-versus-Host Disease
- PMID: 34263142
- PMCID: PMC8274799
- DOI: 10.1097/HS9.0000000000000613
Sphingosine-1-phosphate Receptor-1 Agonist Averts the De Novo Generation of Autoreactive T-cells in Murine Acute Graft-versus-Host Disease
Figures

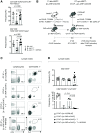
References
-
- Zeiser R, Blazar BR. Acute graft-versus-host disease. N Engl J Med. 2018; 378:585–586. - PubMed
LinkOut - more resources
Full Text Sources
