KDM4 Orchestrates Epigenomic Remodeling of Senescent Cells and Potentiates the Senescence-Associated Secretory Phenotype
- PMID: 34263179
- PMCID: PMC8277122
- DOI: 10.1038/s43587-021-00063-1
KDM4 Orchestrates Epigenomic Remodeling of Senescent Cells and Potentiates the Senescence-Associated Secretory Phenotype
Retraction in
-
Retraction Note: KDM4 orchestrates epigenomic remodeling of senescent cells and potentiates the senescence-associated secretory phenotype.Nat Aging. 2024 Dec;4(12):1898. doi: 10.1038/s43587-024-00749-2. Nat Aging. 2024. PMID: 39482439 No abstract available.
Abstract
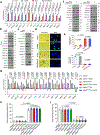
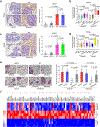
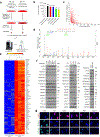
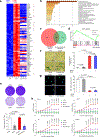
Cellular senescence restrains the expansion of neoplastic cells through several layers of regulation. We report that the histone H3-specific demethylase KDM4 is expressed as human stromal cells undergo senescence. In clinical oncology, upregulated KDM4 and diminished H3K9/H3K36 methylation correlate with poorer survival of prostate cancer patients post-chemotherapy. Global chromatin accessibility mapping via ATAC-seq, and expression profiling through RNA-seq, reveal global changes of chromatin openness and spatiotemporal reprogramming of the transcriptomic landscape, which underlie the senescence-associated secretory phenotype (SASP). Selective targeting of KDM4 dampens the SASP of senescent stromal cells, promotes cancer cell apoptosis in the treatment-damaged tumor microenvironment (TME), and prolongs survival of experimental animals. Our study supports dynamic changes of H3K9/H3K36 methylation during senescence, identifies an unusually permissive chromatin state, and unmasks KDM4 as a key SASP modulator. KDM4 targeting presents a novel therapeutic avenue to manipulate cellular senescence and limit its contribution to age-related pathologies including cancer.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures













Comment in
-
Epiregulation of the SASP makes good neighbors.Nat Aging. 2021 May;1(5):420-421. doi: 10.1038/s43587-021-00068-w. Nat Aging. 2021. PMID: 37118016 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

