AI MSK clinical applications: spine imaging
- PMID: 34263344
- PMCID: PMC8692301
- DOI: 10.1007/s00256-021-03862-0
AI MSK clinical applications: spine imaging
Abstract
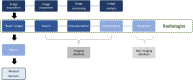
Recent investigations have focused on the clinical application of artificial intelligence (AI) for tasks specifically addressing the musculoskeletal imaging routine. Several AI applications have been dedicated to optimizing the radiology value chain in spine imaging, independent from modality or specific application. This review aims to summarize the status quo and future perspective regarding utilization of AI for spine imaging. First, the basics of AI concepts are clarified. Second, the different tasks and use cases for AI applications in spine imaging are discussed and illustrated by examples. Finally, the authors of this review present their personal perception of AI in daily imaging and discuss future chances and challenges that come along with AI-based solutions.
Keywords: Artificial intelligence; Spine.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Enzmann DR. Radiology’s value chain. Radiology. 2012;263(1):243–252. - PubMed
-
- Gorelik N, Gyftopoulos S. Applications of artificial intelligence in musculoskeletal imaging: from the request to the report. Can Assoc Radiol J. 2021;72(1):45–59. - PubMed
-
- Hirschmann A, Cyriac J, Stieltjes B, Kober T, Richiardi J, Omoumi P. Artificial intelligence in musculoskeletal imaging: review of current literature, challenges, and trends. Semin Musculoskelet Radiol. 2019;23(3):304–311. - PubMed
-
- Mintz Y, Brodie R. Introduction to artificial intelligence in medicine. Minim Invasive Ther Allied Technol. 2019;28(2):73–81. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

