Economic Analyses of Respiratory Tract Infection Diagnostics: A Systematic Review
- PMID: 34263422
- PMCID: PMC8279883
- DOI: 10.1007/s40273-021-01054-1
Economic Analyses of Respiratory Tract Infection Diagnostics: A Systematic Review
Abstract
Background: Diagnostic testing for respiratory tract infections is a tool to manage the current COVID-19 pandemic, as well as the rising incidence of antimicrobial resistance. At the same time, new European regulations for market entry of in vitro diagnostics, in the form of the in vitro diagnostic regulation, may lead to more clinical evidence supporting health-economic analyses.
Objective: The objective of this systematic review was to review the methods used in economic evaluations of applied diagnostic techniques, for all patients seeking care for infectious diseases of the respiratory tract (such as pneumonia, pulmonary tuberculosis, influenza, sinusitis, pharyngitis, sore throats and general respiratory tract infections).
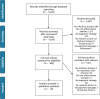
Methods: Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, articles from three large databases of scientific literature were included (Scopus, Web of Science and PubMed) for the period January 2000 to May 2020.
Results: A total of 70 economic analyses are included, most of which use decision tree modelling for diagnostic testing for respiratory tract infections in the community-care setting. Many studies do not incorporate a generally comparable clinical outcome in their cost-effectiveness analysis: fewer than half the studies (33/70) used generalisable outcomes such as quality-adjusted life-years. Other papers consider outcomes related to the accuracy of the test or outcomes related to the prescribed treatment. The time horizons of the studies generally are limited.
Conclusions: The methods to economically assess diagnostic tests for respiratory tract infections vary and would benefit from clear recommendations from policy makers on the assessed time horizon and outcomes used.
© 2021. The Author(s).
Conflict of interest statement
Maarten J. Postma received grants and honoraria from various pharmaceutical companies all unrelated to this research, except those underlying the IMI EU VALUE-Dx project underlying this work. Simon van der Pol, Paula Rojas Garcia, Fernando Antoñanzas Villar and Antoinette D.I. van Asselt have no conflicts of interest that are directly relevant to the content of this article.
Figures
References
-
- Pobre M, Riley R, Predmore Z, Horvitz-Lennon M, Mattke Z. Potential cost savings of a point-of-care diagnostic test measuring antipsychotic plasma levels for treatment of patients with schizophrenia in Spain. Value Health. 2016;19:694.
-
- Salech F, Mery V, Larrondo F, Rada G. Estudios que evalúan un test diagnóstico: interpretando sus resultados. Rev Médica Chile. 2008. p. 136. http://www.scielo.cl/scielo.php?script=sci_arttext&pid=S0034-98872008000.... Accessed 15 May 2020. - PubMed
-
- Di Sanzo M, Cipolloni L, Borro M, La Russa R, Santurro A, Scopetti M, et al. Clinical applications of personalized medicine: a new paradigm and challenge. Curr Pharm Biotechnol. 2017;18:194–203. - PubMed
-
- European Centre for Disease Prevention and Control. COVID-19 testing strategies and objectives. Stockholm; 2020. p. 22. https://www.ecdc.europa.eu/en/publications-data/covid-19-testing-strateg.... Accessed 12 Oct 2020.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical