Efficacy and safety of topical OPA-15406, a new phosphodiesterase 4 inhibitor, in Japanese patients with atopic dermatitis for 8 weeks: A phase 2, randomized, double-blind, placebo-controlled study
- PMID: 34263481
- PMCID: PMC6771806
- DOI: 10.1111/1346-8138.14979
Efficacy and safety of topical OPA-15406, a new phosphodiesterase 4 inhibitor, in Japanese patients with atopic dermatitis for 8 weeks: A phase 2, randomized, double-blind, placebo-controlled study
Abstract
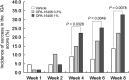
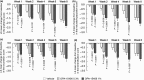
The efficacy and safety of topical OPA-15406, a new phosphodiesterase 4 inhibitor, were examined in Japanese patients aged 15-70 years with atopic dermatitis in a phase 2, randomized, double-blind, vehicle-controlled study. Two hundred patients were randomized to three treatment groups at a 1:1:1 ratio to receive OPA-15406 0.3%, OPA-15406 1% or vehicle ointment twice daily for 8 weeks. The OPA-15406 1% group was superior to the vehicle group in terms of the incidence of success based on the Investigator Global Assessment score at week 4 (P = 0.0328), which was the primary end-point, while the OPA-15406 0.3% group showed a trend toward improvement in the primary end-point. The mean Eczema Area and Severity Index total score and subscale (erythema, induration/papulation, excoriation and lichenification) scores, the Visual Analog Scale pruritus score and the Patient-Oriented Eczema Measure score were significantly improved and the percentage of affected body surface area was significantly decreased in both OPA-15406 groups relative to the vehicle group as early as week 1, and the improved scores and decreased percentages were generally maintained until week 8. No deaths or serious treatment-emergent adverse events occurred in the OPA-15406 treatment groups. Treatment-emergent adverse events frequently observed across treatment groups were worsening of atopic dermatitis, viral upper respiratory tract infection and pruritus, all of which were mild or moderate in severity in the OPA-15406 groups. OPA-15406 1% ointment showed favorable efficacy and safety profiles, indicating a promising treatment option for patients with atopic dermatitis.
Keywords: OPA‐15406; atopic dermatitis; phosphodiesterase 4 inhibitor; pruritus; topical.
© 2019 The Authors. The Journal of Dermatology published by John Wiley & Sons Australia, Ltd on behalf of Japanese Dermatological Association.
Conflict of interest statement
This study was supported by Otsuka Pharmaceutical, and all study drugs used in this study were provided by Otsuka Pharmaceutical. H. S. was the medical expert for the study and M. K. was the coordinating investigator at study sites. H. S. and M. K. have received fees for consultation from Otsuka Pharmaceutical. S. S., K. O. and H. T. are employees of Otsuka Pharmaceutical.
Figures
References
-
- Nygaard U, Deleuran M, Vestergaard C. Emerging treatment options in atopic dermatitis: topical therapies. Dermatology 2017; 233: 333–343. - PubMed
-
- Simpson EL, Bieber T, Eckert L et al Patient burden of moderate to severe atopic dermatitis (AD): insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol 2016; 74: 491–498. - PubMed
-
- Narla S, Hsu DY, Thyssen JP, Silverberg JI. Inpatient financial burden of atopic dermatitis in the United States. J Invest Dermatol 2017; 137: 1461–1467. - PubMed
-
- Ministry of Health, Labour and Welfare . Summary of patient survey 2014 [Cited 13 Feb 2019]. Available from URL: http://www.mhlw.go.jp/toukei/saikin/hw/kanja/10syoubyo/.
Grants and funding
LinkOut - more resources
Full Text Sources

