Discriminatory plasma biomarkers predict specific clinical phenotypes of necrotizing soft-tissue infections
- PMID: 34263738
- PMCID: PMC8279592
- DOI: 10.1172/JCI149523
Discriminatory plasma biomarkers predict specific clinical phenotypes of necrotizing soft-tissue infections
Abstract
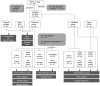
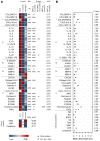
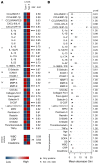
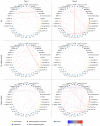
BACKGROUNDNecrotizing soft-tissue infections (NSTIs) are rapidly progressing infections frequently complicated by septic shock and associated with high mortality. Early diagnosis is critical for patient outcome, but challenging due to vague initial symptoms. Here, we identified predictive biomarkers for NSTI clinical phenotypes and outcomes using a prospective multicenter NSTI patient cohort.METHODSLuminex multiplex assays were used to assess 36 soluble factors in plasma from NSTI patients with positive microbiological cultures (n = 251 and n = 60 in the discovery and validation cohorts, respectively). Control groups for comparative analyses included surgical controls (n = 20), non-NSTI controls (i.e., suspected NSTI with no necrosis detected upon exploratory surgery, n = 20), and sepsis patients (n = 24).RESULTSThrombomodulin was identified as a unique biomarker for detection of NSTI (AUC, 0.95). A distinct profile discriminating mono- (type II) versus polymicrobial (type I) NSTI types was identified based on differential expression of IL-2, IL-10, IL-22, CXCL10, Fas-ligand, and MMP9 (AUC >0.7). While each NSTI type displayed a distinct array of biomarkers predicting septic shock, granulocyte CSF (G-CSF), S100A8, and IL-6 were shared by both types (AUC >0.78). Finally, differential connectivity analysis revealed distinctive networks associated with specific clinical phenotypes.CONCLUSIONSThis study identifies predictive biomarkers for NSTI clinical phenotypes of potential value for diagnostic, prognostic, and therapeutic approaches in NSTIs.TRIAL REGISTRATIONClinicalTrials.gov NCT01790698.FUNDINGCenter for Innovative Medicine (CIMED); Region Stockholm; Swedish Research Council; European Union; Vinnova; Innovation Fund Denmark; Research Council of Norway; Netherlands Organisation for Health Research and Development; DLR Federal Ministry of Education and Research; and Swedish Children's Cancer Foundation.
Keywords: Bacterial infections; Diagnostics; Immunology; Infectious disease.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

