Capture and delivery of tail-anchored proteins to the endoplasmic reticulum
- PMID: 34264263
- PMCID: PMC8287540
- DOI: 10.1083/jcb.202105004
Capture and delivery of tail-anchored proteins to the endoplasmic reticulum
Abstract
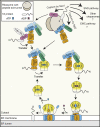
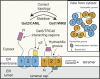
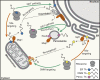
Tail-anchored (TA) proteins fulfill diverse cellular functions within different organellar membranes. Their characteristic C-terminal transmembrane segment renders TA proteins inherently prone to aggregation and necessitates their posttranslational targeting. The guided entry of TA proteins (GET in yeast)/transmembrane recognition complex (TRC in humans) pathway represents a major route for TA proteins to the endoplasmic reticulum (ER). Here, we review important new insights into the capture of nascent TA proteins at the ribosome by the GET pathway pretargeting complex and the mechanism of their delivery into the ER membrane by the GET receptor insertase. Interestingly, several alternative routes by which TA proteins can be targeted to the ER have emerged, raising intriguing questions about how selectivity is achieved during TA protein capture. Furthermore, mistargeting of TA proteins is a fundamental cellular problem, and we discuss the recently discovered quality control machineries in the ER and outer mitochondrial membrane for displacing mislocalized TA proteins.
© 2021 Farkas and Bohnsack.
Figures

References
-
- Asseck, L.Y., Mehlhorn D.G., Monroy J.R., Ricardi M.M., Breuninger H., Wallmeroth N., Berendzen K.W., Nowrousian M., Xing S., Schwappach B., et al. 2021. Endoplasmic reticulum membrane receptors of the GET pathway are conserved throughout eukaryotes. Proc. Natl. Acad. Sci. USA. 118:e2017636118. 10.1073/pnas.2017636118 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

