High-Dose Buprenorphine Induction in the Emergency Department for Treatment of Opioid Use Disorder
- PMID: 34264326
- PMCID: PMC8283555
- DOI: 10.1001/jamanetworkopen.2021.17128
High-Dose Buprenorphine Induction in the Emergency Department for Treatment of Opioid Use Disorder
Abstract
Importance: Emergency departments (EDs) sporadically use a high-dose buprenorphine induction strategy for the treatment of opioid use disorder (OUD) in response to the increasing potency of the illicit opioid drug supply and commonly encountered delays in access to follow-up care.
Objective: To examine the safety and tolerability of high-dose (>12 mg) buprenorphine induction for patients with OUD presenting to an ED.
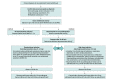
Design, setting, and participants: In this case series of ED encounters, data were manually abstracted from electronic health records for all ED patients with OUD treated with buprenorphine at a single, urban, safety-net hospital in Oakland, California, for the calendar year 2018. Data analysis was performed from April 2020 to March 2021.
Interventions: ED physicians and advanced practice practitioners were trained on a high-dose sublingual buprenorphine induction protocol, which was then clinically implemented.
Main outcomes and measures: Vital signs; use of supplemental oxygen; the presence of precipitated withdrawal, sedation, and respiratory depression; adverse events; length of stay; and hospitalization during and 24 hours after the ED visit were reported according to total sublingual buprenorphine dose (range, 2 to >28 mg).
Results: Among a total of 391 unique patients (median [interquartile range] age, 36 [29-48] years), representing 579 encounters, 267 (68.3%) were male and 170 were (43.5%) Black. Homelessness (88 patients [22.5%]) and psychiatric disorders (161 patients [41.2%]) were common. A high dose of sublingual buprenorphine (>12 mg) was administered by 54 unique clinicians during 366 (63.2%) encounters, including 138 doses (23.8%) greater than or equal to 28 mg. No cases of respiratory depression or sedation were reported. All 5 (0.8%) cases of precipitated withdrawal had no association with dose; 4 cases occurred after doses of 8 mg of buprenorphine. Three serious adverse events unrelated to buprenorphine were identified. Nausea or vomiting was rare (2%-6% of cases). The median (interquartile range) length of stay was 2.4 (1.6-3.75) hours.
Conclusions and relevance: These findings suggest that high-dose buprenorphine induction, adopted by multiple clinicians in a single-site urban ED, was safe and well tolerated in patients with untreated OUD. Further prospective investigations conducted in multiple sites would enhance these findings.
Conflict of interest statement
Figures


References
-
- Ahrnsbrak R, Bose J, Hedden SL, Lipari RN, Park-Lee E; Substance Abuse and Mental Health Administration . Key substance use and mental health indicators in the United States: results from the 2016 National Survey on Drug Use and Health (HHS publication No. SMA 17-5044, NSDUH series H-52). Published September 2017. Accessed June 7, 2021. https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2016/NSDUH-FF...
-
- National Institute on Drug Abuse . Opioid overdose crisis. Published 2020. Updated March 11, 2021. Accessed June 1, 2020. https://www.drugabuse.gov/drugs-topics/opioids/opioid-overdose-crisis
-
- National Academies of Medicine, Science, and Engineering . Medications for Opioid Use Disorder Save Lives. The National Academies Press; 2019. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

