An artificial intelligence-assisted diagnostic platform for rapid near-patient hematology
- PMID: 34264525
- PMCID: PMC9290600
- DOI: 10.1002/ajh.26295
An artificial intelligence-assisted diagnostic platform for rapid near-patient hematology
Abstract
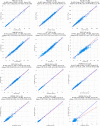
Hematology analyzers capable of performing complete blood count (CBC) have lagged in their prevalence at the point-of-care. Sight OLO (Sight Diagnostics, Israel) is a novel hematological platform which provides a 19-parameter, five-part differential CBC, and is designed to address the limitations in current point-of-care hematology analyzers using recent advances in artificial intelligence (AI) and computer vision. Accuracy, repeatability, and flagging capabilities of OLO were compared with the Sysmex XN-Series System (Sysmex, Japan). Matrix studies compared performance using venous, capillary and direct-from-fingerprick blood samples. Regression analysis shows strong concordance between OLO and the Sysmex XN, demonstrating that OLO performs with high accuracy for all CBC parameters. High repeatability and reproducibility were demonstrated for most of the testing parameters. The analytical performance of the OLO hematology analyzer was validated in a multicenter clinical laboratory setting, demonstrating its accuracy and comparability to clinical laboratory-based hematology analyzers. Furthermore, the study demonstrated the validity of CBC analysis of samples collected directly from fingerpricks.
© 2021 The Authors. American Journal of Hematology published by Wiley Periodicals LLC.
Conflict of interest statement
Employment or Leadership: Yochay Eshel, S.D. Sight Diagnostics LTD; Daniel Levner, S.D. Sight Diagnostics LTD; Amir Zait, S.D. Sight Diagnostics LTD; Dan Glück, S.D. Sight Diagnostics LTD; Dana Benbassat, S.D. Sight Diagnostics LTD; Sharon Pecker, S.D. Sight Diagnostics LTD; David Brailovsky, S.D. Sight Diagnostics LTD; Evgeny Yurkovsky, S.D. Sight Diagnostics LTD; Neta Bachar, S.D. Sight Diagnostics LTD; Sarah Levy, S.D. Sight Diagnostics LTD. Cordelia Sever, Alexander Kratz, and Carlo Brugnara received research funding from S.D. Sight Diagnostics LTD in the form of a sponsored research agreement/contract. Yochay Eshel, Daniel Levner, Amir Zait, Dan Glück, Dana Benbassat, Sharon Pecker, David Brailovsky, Evgeny Yurkovsky, Neta Bachar, and Sarah Levyare S.D. Sight Diagnostics LTD employees and stockholders.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
