Cycle threshold values in RT-PCR to determine dynamics of SARS-CoV-2 viral load: An approach to reduce the isolation period for COVID-19 patients
- PMID: 34264527
- PMCID: PMC8426941
- DOI: 10.1002/jmv.27206
Cycle threshold values in RT-PCR to determine dynamics of SARS-CoV-2 viral load: An approach to reduce the isolation period for COVID-19 patients
Abstract
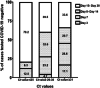
Severe acute respiratory syndrome coronavirus (SARS-CoV-2) has affected all inhabited continents, and India is currently experiencing a devastating second wave of coronavirus disease-2019 (COVID-19). Here, we examined the duration of clearance of SARS-CoV-2 in respiratory samples from 207 infected cases by real-time reverse-transcription polymerase chain reaction (RT-PCR). A substantial proportion of COVID-19 positive cases with cycle threshold (Ct) values more than or equal to 31 (45.7%) were subsequently tested negative for SARS-CoV-2 RNA within 7 days of initial detection of the viral load. A total of 60% of all the patients with COVID-19, irrespective of their Ct values, cleared SARS-CoV-2 RNA within 14 days of the initial detection. Longitudinal assessment of RT-PCR test results in individuals requiring 15-30 days to clear SARS-CoV-2 RNA showed a significant reduction of the viral load in samples with high or intermediate viral loads (Ct values ≤ 25 and between 26 and 30, respectively) but the follow-up group with low viral RNA (Ct values ≥ 31) exhibited a stable viral load. Together, these results suggest that COVID-19 positive cases with Ct values more than or equal to 31 require reduced duration to clear SARS-CoV-2, and thus, a shorter isolation period for this group might be considered to facilitate adequate space in the COVID Care Centres and reduce the burden on healthcare infrastructure.
Keywords: COVID 19; RT-PCR; SARS-CoV-2; cycle threshold value; isolation.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

References
-
- Biguenet A, Bouiller K, Marty‐Quinternet S, Brunel AS, Chirouze C, Lepiller Q. SARS‐CoV‐2 respiratory viral loads and association with clinical and biological features. J Med Virol. 2021;93(3):1761‐1765. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

