Is severe COVID-19 a cytokine storm syndrome: a hyperinflammatory debate
- PMID: 34264880
- PMCID: PMC8373392
- DOI: 10.1097/BOR.0000000000000822
Is severe COVID-19 a cytokine storm syndrome: a hyperinflammatory debate
Abstract
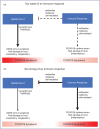
Purpose of review: The COVID-19 pandemic is a global public health crisis with considerable mortality and morbidity. A role for cytokine storm and therapeutic immunomodulation in a subgroup of patients with severe COVID-19 was proposed early in the pandemic. The concept of cytokine storm in COVID-19 has been criticised, given the lack of clear definition and relatively modest cytokinaemia (which may be necessary for viral clearance) compared with acute respiratory distress syndrome and bacterial sepsis. Here we consider the arguments for and against the concept of cytokine storm in COVID-19.
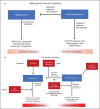
Recent findings: Several criteria have been proposed to identify the subgroup of COVID-19 patients exhibiting a cytokine storm. The beneficial effects of corticosteroids and interleukin-6 inhibition suggest that inflammation is a modifiable pathogenic component of severe COVID-19. The presence of genetic polymorphisms and pathogenic auto-autoantibodies in severe COVID-19 also suggests a significant contribution of immune dysregulation to poor outcomes.
Summary: Hyperinflammation is a key component of severe COVID-19, residing underneath the cytokine storm umbrella term, associated with poor outcomes. Better understanding of the aetiopathogenesis, with identification of biomarkers to predict treatment responses and prognosis, will hopefully enable a stratified and ultimately precision medicine approach.
Copyright © 2021 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

