SARS-CoV-2 B.1.617.2 (Delta) Variant COVID-19 Outbreak Associated with a Gymnastics Facility - Oklahoma, April-May 2021
- PMID: 34264910
- PMCID: PMC8314708
- DOI: 10.15585/mmwr.mm7028e2
SARS-CoV-2 B.1.617.2 (Delta) Variant COVID-19 Outbreak Associated with a Gymnastics Facility - Oklahoma, April-May 2021
Abstract
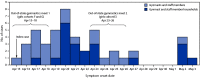
The B.1.617.2 (Delta) variant of SARS-CoV-2, the virus that causes COVID-19, was identified in India in late 2020 and has subsequently been detected in approximately 60 countries (1). The B.1.617.2 variant has a potentially higher rate of transmission than other variants (2). During May 12-18, 2021, the Oklahoma State Department of Health (OSDH) Acute Disease Service (ADS) was notified by the OSDH Public Health Laboratory (PHL) of 21 SARS-CoV-2 B.1.617.2 specimens temporally and geographically clustered in central Oklahoma. Public health surveillance data indicated that these cases were associated with a local gymnastics facility (facility A). OSDH ADS and local health department staff members reinterviewed persons with B.1.617.2 variant-positive laboratory results and conducted contact tracing. Forty-seven COVID-19 cases (age range = 5-58 years), including 21 laboratory-confirmed B.1.617.2 variant and 26 epidemiologically linked cases, were associated with this outbreak during April 15-May 3, 2021. Cases occurred among 10 of 16 gymnast cohorts* and three staff members; secondary cases occurred in seven (33%) of 26 interviewed households with outbreak-associated cases. The overall facility and household attack rates were 20% and 53%, respectively. Forty (85%) persons with outbreak-associated COVID-19 had never received any COVID-19 vaccine doses (unvaccinated); three (6%) had received 1 dose of Moderna or Pfizer-BioNTech ≥14 days before a positive test result but had not received the second dose (partially vaccinated); four persons (9%) had received 2 doses of Moderna or Pfizer-BioNTech or a single dose of Janssen (Johnson & Johnson) vaccine ≥14 days before a positive test result (fully vaccinated). These findings suggest that the B.1.617.2 variant is highly transmissible in indoor sports settings and within households. Multicomponent prevention strategies including vaccination remain important to reduce the spread of SARS-CoV-2, including among persons participating in indoor sports† and their contacts.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
References
-
- CDC. COVID-19: SARS-CoV-2 variant classifications and definitions. Atlanta, GA: US Department of Health and Human Services, CDC; 2021. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/variant-surveill...
-
- Public Health England. SARS-CoV-2 variants of concern and variants under investigation in England—technical briefing 17. London, United Kingdom: Public Health England; 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploa...
-
- Illumina. Illumina COVIDSeq test instructions for use. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2020. Accessed June 4, 2021. https://www.fda.gov/media/138776/download
-
- Public Health England. SARS-CoV-2 variants of concern and variants under investigation in England—technical briefing 13. London, United Kingdom: Public Health England; 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploa...
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous