Effect of Postoperative Radiotherapy after Primary Tumor Resection in De Novo Stage IV Breast Cancer: A Multicenter Retrospective Study (KROG 19-02)
- PMID: 34265890
- PMCID: PMC9016291
- DOI: 10.4143/crt.2021.632
Effect of Postoperative Radiotherapy after Primary Tumor Resection in De Novo Stage IV Breast Cancer: A Multicenter Retrospective Study (KROG 19-02)
Abstract
Purpose: This study aimed to investigate the impact of postoperative radiotherapy (PORT) in de novo metastatic breast cancer (dnMBC) patients undergoing planned primary tumor resection (PTR) and to identify the subgroup of patients who would most benefit from PORT.
Materials and methods: This study enrolled 426 patients with dnMBC administered PTR alone or with PORT. The primary and secondary outcomes were overall and progression-free survival (OS and PFS), respectively.
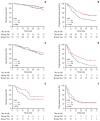
Results: The median follow-up time was 53.7 months (range, 3.1 to 194.4). The 5-year OS and PFS rates were 73.2% and 32.0%, respectively. For OS, clinical T3/4 category, triple-negative breast cancer (TNBC), postoperative chemotherapy alone were significantly poor prognostic factors, and administration of PORT failed to show its significance. Regarding PFS, PORT was a favorable prognostic factor (hazard ratio, 0.64; 95% confidence interval, 0.50 to 0.82; p < 0.001), in addition to T1/2 category, ≤ 5 metastases, and non-TNBC. According to the multivariate analyses of OS in the PORT group, we divided the patients into three groups (group 1, T1/2 and non-TNBC [n=193]; group 2, T3/4 and non-TNBC [n=171]; and group 3, TNBC [n=49]), and evaluated the effect of PORT. Although PORT had no significance for OS in all subgroups, it was a significant factor for good prognosis regarding PFS in groups 1 and 2, not in group 3.
Conclusion: PORT was associated with a significantly better PFS in patients with dnMBC who underwent PTR. Patients with clinical T1/2 category and non-TNBC benefited most from PORT, while those with TNBC showed little benefit.
Keywords: Breast surgery; Postoperative radiotherapy; Stage IV breast cancer; Survival.
Conflict of interest statement
Conflict of interest relevant to this article was not reported.
Figures
References
-
- DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66:31–42. - PubMed
-
- Chia SK, Speers CH, D’Yachkova Y, Kang A, Malfair-Taylor S, Barnett J, et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer. 2007;110:973–9. - PubMed
-
- Sundquist M, Brudin L, Tejler G. Improved survival in metastatic breast cancer 1985–2016. Breast. 2017;31:46–50. - PubMed
-
- Badwe R, Hawaldar R, Nair N, Kaushik R, Parmar V, Siddique S, et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet Oncol. 2015;16:1380–8. - PubMed
-
- Soran A, Ozmen V, Ozbas S, Karanlik H, Muslumanoglu M, Igci A, et al. Randomized trial comparing resection of primary tumor with no surgery in stage IV breast cancer at presentation: protocol MF07-01. Ann Surg Oncol. 2018;25:3141–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources