Multispectral fluorescence lifetime imaging device with a silicon avalanche photodetector
- PMID: 34266107
- PMCID: PMC8237936
- DOI: 10.1364/OE.425632
Multispectral fluorescence lifetime imaging device with a silicon avalanche photodetector
Abstract
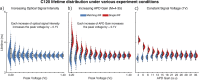
We report the design, development, and characterization of a novel multi-spectral fluorescence lifetime measurement device incorporating solid-state detectors and automated gain control. For every excitation pulse (∼1 µJ, 600 ps), this device records complete fluorescence decay from multiple spectral channels simultaneously within microseconds, using a dedicated UV enhanced avalanche photodetector and analog to digital convert (2.5 GS/s) in each channel. Fast (<2 ms) channel-wise dynamic range adjustment maximizes the signal-to-noise ratio. Fluorophores with known lifetime ranging from 0.5-6.0 ns were used to demonstrate the device accuracy. Current results show the clear benefits of this device compared to existing devices employing microchannel-plate photomultiplier tubes. This is demonstrated by 5-fold reduction of lifetime measurement variability in identical conditions, independent gain adjustment in each spectral band, and 4-times faster imaging speed. The use of solid-state detectors will also facilitate future improved performance and miniaturization of the instrument.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Coda S., Thompson A. J., Kennedy G. T., Roche K. L., Ayaru L., Bansi D. S., Stamp G. W., Thillainayagam A. V., French P. M., Dunsby C., “Fluorescence lifetime spectroscopy of tissue autofluorescence in normal and diseased colon measured ex vivo using a fiber-optic probe,” Biomed. Opt. Express 5(2), 515–538 (2014).10.1364/BOE.5.000515 - DOI - PMC - PubMed
-
- Malik B. H., Lee J., Cheng S., Cuenca R., Jabbour J. M., Cheng Y. S., Wright J. M., Ahmed B., Maitland K. C., Jo J. A., “Objective Detection of Oral Carcinoma with Multispectral Fluorescence Lifetime Imaging In Vivo,” Photochem. Photobiol. 92(5), 694–701 (2016).10.1111/php.12627 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

