Mechanisms of O2 Activation by Mononuclear Non-Heme Iron Enzymes
- PMID: 34266238
- PMCID: PMC8768060
- DOI: 10.1021/acs.biochem.1c00370
Mechanisms of O2 Activation by Mononuclear Non-Heme Iron Enzymes
Abstract
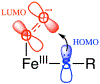
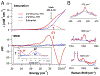
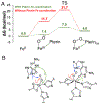
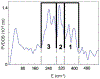
Two major subclasses of mononuclear non-heme ferrous enzymes use two electron-donating organic cofactors (α-ketoglutarate or pterin) to activate O2 to form FeIV═O intermediates that further react with their substrates through hydrogen atom abstraction or electrophilic aromatic substitution. New spectroscopic methodologies have been developed, enabling the study of the active sites in these enzymes and their oxygen intermediates. Coupled to electronic structure calculations, the results of these spectroscopies provide fundamental insight into mechanism. This Perspective summarizes the results of these studies in elucidating the mechanism of dioxygen activation to form the FeIV═O intermediate and the geometric and electronic structure of this intermediate that enables its high reactivity and selectivity in product formation.
Figures

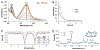
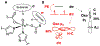

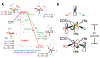


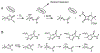
References
-
- Baldwin JE, and Abraham E (1988) The Biosynthesis of Penicillins and Cephalosporins. Natural Product Reports 5, 129–145. - PubMed
-
- Busby RW, and Townsend CA (1996) A single monomeric iron center in clavaminate synthase catalyzes three nonsuccessive oxidative transformations. Bioorganic Medicinal Chemistry 4, 1059–1064. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

