Effect of exacerbation history on clinical response to dupilumab in moderate-to-severe uncontrolled asthma
- PMID: 34266940
- PMCID: PMC8551561
- DOI: 10.1183/13993003.04498-2020
Effect of exacerbation history on clinical response to dupilumab in moderate-to-severe uncontrolled asthma
Abstract
Background: The phase 3 LIBERTY ASTHMA QUEST study (ClinicalTrials.gov: NCT02414854) in patients with uncontrolled, moderate-to-severe asthma has demonstrated the efficacy and safety of dupilumab 200 and 300 mg every 2 weeks versus placebo. This post hoc analysis assessed the effect of dupilumab on efficacy outcomes and asthma control across a range of historical exacerbation rates in patients with type 2-high asthma.
Methods: Annualised severe exacerbation rates over the 52-week treatment period, pre-bronchodilator forced expiratory volume in 1 s (FEV1) at weeks 12 and 52, and the five-item Asthma Control Questionnaire (ACQ-5) score at weeks 24 and 52 were assessed in patients with ≥1, ≥2 or ≥3 exacerbations in the previous year. Subgroups were stratified by baseline blood eosinophils ≥150 or ≥300 cells·μL-1 or baseline exhaled nitric oxide fraction ≥25 ppb and baseline inhaled corticosteroid (ICS) dose.
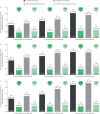
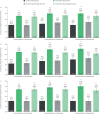
Results: Across all type 2-high subgroups, dupilumab versus placebo significantly reduced severe exacerbations by 54-90%, with greater improvements in patients with more exacerbations prior to study initiation. Similarly, improvements in FEV1 (least squares (LS) mean difference versus placebo: ≥1 exacerbations, 0.15-0.25 L; ≥2 exacerbations, 0.12-0.32 L; ≥3 exacerbations, 0.09-0.38 L; majority p<0.05) and ACQ-5 score (LS mean difference range: ≥1 exacerbations, -0.30 to -0.57; ≥2 exacerbations, -0.29 to -0.56; ≥3 exacerbations, -0.43 to -0.61; all p<0.05) were observed, irrespective of prior exacerbation history, across all subgroups.
Conclusions: Dupilumab significantly reduced severe exacerbations and improved FEV1 and asthma control in patients with elevated type 2 biomarkers irrespective of exacerbation history and baseline ICS dose.
Copyright ©The authors 2021.
Conflict of interest statement
Conflict of interest: J. Corren reports nonfinancial support for research from Sanofi, outside the submitted work. Conflict of interest: C.H. Katelaris reports nonfinancial support (advisory board member and principal study investigator) from Sanofi, outside the submitted work. Conflict of interest: M. Castro reports nonfinancial support for research from the American Lung Association, Gossamer, NIH, PCORI and Shionogi, personal fees for lectures and nonfinancial support for research from AstraZeneca, personal fees for lectures and consultancy, and nonfinancial support for research from Boehringer Ingelheim and Sanofi, personal fees for consultancy and nonfinancial support for research from Novartis, personal fees for consultancy from Boston Scientific and Vida Pharma, personal fees for lectures and consultancy from Teva, personal fees for lectures from Genentech and Regeneron Pharmaceuticals, Inc., personal fees (royalties) from Elsevier, outside the submitted work. Conflict of interest: J.F. Maspero reports being a speaker for AstraZeneca, GlaxoSmithKline, Menarini, Novartis, Sanofi, Teva and Uriach; and has received research grants from Novartis, outside the submitted work. Conflict of interest: L.B. Ford reports nonfinancial support for research from AstraZeneca, DBV, Genentech, Gossamer, Novartis and Teva, personal fees for consultancy from Sanofi, outside the submitted work. Conflict of interest: D.M.G. Halpin reports personal fees for lectures and advisory board work from AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, GlaxoSmithKline, Novartis, Pfizer, Sandoz and Sanofi, outside the submitted work. Conflict of interest: M.S. Rice is an employee of Sanofi and may hold stock and/or stock options in the company. Conflict of interest: A. Radwan is an employee and shareholder of Regeneron Pharmaceuticals, Inc. Conflict of interest: Y. Deniz is an employee and shareholder of Regeneron Pharmaceuticals, Inc. Conflict of interest: P.J. Rowe is an employee of Sanofi and may hold stock and/or stock options in the company. Conflict of interest: A. Teper is a former employee of Sanofi. Conflict of interest: M. Djandji is an employee of Sanofi and may hold stock and/or stock options in the company.
Figures