Role of NMDA Receptors in Adult Neurogenesis and Normal Development of the Dentate Gyrus
- PMID: 34266965
- PMCID: PMC8354713
- DOI: 10.1523/ENEURO.0566-20.2021
Role of NMDA Receptors in Adult Neurogenesis and Normal Development of the Dentate Gyrus
Abstract
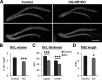
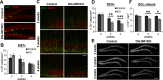
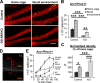
The NMDA receptors are a type of glutamate receptors, which is involved in neuronal function, plasticity and development in the mammalian brain. However, how the NMDA receptors contribute to adult neurogenesis and development of the dentate gyrus is unclear. In this study, we investigate this question by examining a region-specific knock-out mouse line that lacks the NR1 gene, which encodes the essential subunit of the NMDA receptors, in granule cells of the dentate gyrus (DG-NR1KO mice). We found that the survival of newly-generated granule cells, cell proliferation and the size of the granule cell layer are significantly reduced in the dorsal dentate gyrus of adult DG-NR1KO mice. Our results also show a significant reduction in the number of immature neurons and in the volume of the granule cell layer, starting from three weeks of postnatal age. DG-NR1KO mice also showed impairment in the expression of an immediate early gene, Arc, and behavior during the novelty-suppressed feeding and open field test. These results suggest that the NMDA receptors in granule cells have a role in adult neurogenesis in the adult brain and contributes to the normal development of the dentate gyrus.
Keywords: granule cell; hippocampus; neuronal survival; novelty suppressed feeding test; pattern separation.
Copyright © 2021 Åmellem et al.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
