Females, but not males, require protein degradation in the hippocampus for contextual fear memory formation
- PMID: 34266989
- PMCID: PMC8284313
- DOI: 10.1101/lm.053429.121
Females, but not males, require protein degradation in the hippocampus for contextual fear memory formation
Abstract
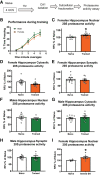
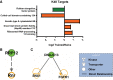
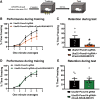
Strong evidence supports a role for protein degradation in fear memory formation. However, these data have been largely done in only male animals. Here, we found that following contextual fear conditioning, females, but not males, had increased levels of proteasome activity and K48 polyubiquitin protein targeting in the dorsal hippocampus, the latter of which occurred at chaperones or RNA processing proteins. In vivo CRISPR-dCas9-mediated repression of protein degradation in the dorsal hippocampus impaired contextual fear memory in females, but not males. These results suggest a sex-specific role for protein degradation in the hippocampus during the consolidation of a contextual fear memory.
© 2021 Martin et al.; Published by Cold Spring Harbor Laboratory Press.
Figures
Similar articles
-
Proteomic Analysis Reveals Sex-Specific Protein Degradation Targets in the Amygdala During Fear Memory Formation.Front Mol Neurosci. 2021 Sep 29;14:716284. doi: 10.3389/fnmol.2021.716284. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34658783 Free PMC article.
-
Males and females differ in the regulation and engagement of, but not requirement for, protein degradation in the amygdala during fear memory formation.Neurobiol Learn Mem. 2021 Apr;180:107404. doi: 10.1016/j.nlm.2021.107404. Epub 2021 Feb 18. Neurobiol Learn Mem. 2021. PMID: 33609735 Free PMC article.
-
Decreases in K63 Polyubiquitination in the Hippocampus Promote the Formation of Contextual Fear Memories in Both Males and Females.Hippocampus. 2025 Jan;35(1):e23650. doi: 10.1002/hipo.23650. Hippocampus. 2025. PMID: 39610227
-
An update on contextual fear memory mechanisms: Transition between Amygdala and Hippocampus.Neurosci Biobehav Rev. 2018 Sep;92:43-54. doi: 10.1016/j.neubiorev.2018.05.013. Epub 2018 May 9. Neurosci Biobehav Rev. 2018. PMID: 29752958 Review.
-
Hippocampus and contextual fear conditioning: recent controversies and advances.Hippocampus. 2001;11(1):8-17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. Hippocampus. 2001. PMID: 11261775 Review.
Cited by
-
The ubiquitin-proteasome system and learning-dependent synaptic plasticity - A 10 year update.Neurosci Biobehav Rev. 2023 Sep;152:105280. doi: 10.1016/j.neubiorev.2023.105280. Epub 2023 Jun 12. Neurosci Biobehav Rev. 2023. PMID: 37315660 Free PMC article. Review.
-
Windows of change: Revisiting temporal and molecular dynamics of memory reconsolidation and persistence.Neurosci Biobehav Rev. 2025 Jul;174:106198. doi: 10.1016/j.neubiorev.2025.106198. Epub 2025 May 10. Neurosci Biobehav Rev. 2025. PMID: 40354954 Review.
-
The epigenetic role of proteasome subunit RPT6 during memory formation in female rats.Learn Mem. 2022 Sep 2;29(9):256-264. doi: 10.1101/lm.053498.121. Print 2022 Sep. Learn Mem. 2022. PMID: 36206393 Free PMC article.
-
Increasing degradation-independent linear polyubiquitin in the hippocampus enhances memory in young adult but not aged rats.Neurobiol Learn Mem. 2025 Jul;220:108075. doi: 10.1016/j.nlm.2025.108075. Epub 2025 Jun 20. Neurobiol Learn Mem. 2025. PMID: 40544948
-
Sex differences in training-induced activity of the ubiquitin proteasome system in the dorsal hippocampus and medial prefrontal cortex of male and female mice.Learn Mem. 2022 Sep 2;29(9):302-311. doi: 10.1101/lm.053492.121. Print 2022 Sep. Learn Mem. 2022. PMID: 36206392 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
