Safety and immunogenicity of a recombinant COVID-19 vaccine (Sf9 cells) in healthy population aged 18 years or older: two single-center, randomised, double-blind, placebo-controlled, phase 1 and phase 2 trials
- PMID: 34267185
- PMCID: PMC8281021
- DOI: 10.1038/s41392-021-00692-3
Safety and immunogenicity of a recombinant COVID-19 vaccine (Sf9 cells) in healthy population aged 18 years or older: two single-center, randomised, double-blind, placebo-controlled, phase 1 and phase 2 trials
Abstract
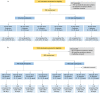
COVID-19 vaccines from multiple manufacturers are needed to cope with the problem of insufficient supply. We did two single-center, randomised, double-blind, placebo-controlled phase 1 and phase 2 trials to assess the safety, tolerability and immunogenicity of a recombinant COVID-19 vaccine (Sf9 cells) in healthy population aged 18 years or older in China. Eligible participants were enrolled, the ratio of candidate vaccine and placebo within each dose group was 3:1 (phase 1) or 5:1 (phase 2). From August 28, 2020, 168 participants were sequentially enrolled and randomly assigned to receive the low dose vaccine, high dose vaccine or placebo with the schedule of 0, 28 days or 0, 14, 28 days in phase 1 trial. From November 18, 2020, 960 participants were randomly assigned to receive the low dose vaccine, high dose vaccine or placebo with the schedule of 0, 21 days or 0, 14, 28 days in phase 2 trial. The most common solicited injection site adverse reaction within 7 days in both trials was pain. The most common solicited systematic adverse reactions within 7 days were fatigue, cough, sore throat, fever and headache. ELISA antibodies and neutralising antibodies increased at 14 days, and peaked at 28 days (phase 1) or 30 days (phase 2) after the last dose vaccination. The GMTs of neutralising antibody against live SARS-CoV-2 at 28 days or 30 days after the last dose vaccination were highest in the adult high dose group (0, 14, 28 days), with 102.9 (95% CI 61.9-171.2) and 102.6 (95% CI 75.2-140.1) in phase 1 and phase 2 trials, respectively. Specific T-cell response peaked at 14 days after the last dose vaccination in phase 1 trial. This vaccine is safe, and induced significant immune responses after three doses of vaccination.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- WHO. WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int (2021).
-
- WHO. Draft landscape and tracker of COVID-19 candidate vaccines. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand... (2021).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

