Chimeric antigen receptor- and natural killer cell receptor-engineered innate killer cells in cancer immunotherapy
- PMID: 34267335
- PMCID: PMC8429625
- DOI: 10.1038/s41423-021-00732-6
Chimeric antigen receptor- and natural killer cell receptor-engineered innate killer cells in cancer immunotherapy
Abstract
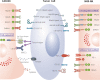
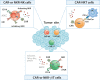
Chimeric antigen receptor (CAR)-engineered T-cell (CAR-T) therapy has demonstrated impressive therapeutic efficacy against hematological malignancies, but multiple challenges have hindered its application, particularly for the eradication of solid tumors. Innate killer cells (IKCs), particularly NK cells, NKT cells, and γδ T cells, employ specific antigen-independent innate tumor recognition and cytotoxic mechanisms that simultaneously display high antitumor efficacy and prevent tumor escape caused by antigen loss or modulation. IKCs are associated with a low risk of developing GVHD, thus offering new opportunities for allogeneic "off-the-shelf" cellular therapeutic products. The unique innate features, wide tumor recognition range, and potent antitumor functions of IKCs make them potentially excellent candidates for cancer immunotherapy, particularly serving as platforms for CAR development. In this review, we first provide a brief summary of the challenges hampering CAR-T-cell therapy applications and then discuss the latest CAR-NK-cell research, covering the advantages, applications, and clinical translation of CAR- and NK-cell receptor (NKR)-engineered IKCs. Advances in synthetic biology and the development of novel genetic engineering techniques, such as gene-editing and cellular reprogramming, will enable the further optimization of IKC-based anticancer therapies.
Keywords: Adoptive cell therapy; Chimeric antigen receptor; Genetic engineering; Innate killer cells; Natural killer cell receptor; Tumor microenvironment.
© 2021. The Author(s), under exclusive licence to CSI and USTC.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

