The basal ganglia control the detailed kinematics of learned motor skills
- PMID: 34267392
- PMCID: PMC11152194
- DOI: 10.1038/s41593-021-00889-3
The basal ganglia control the detailed kinematics of learned motor skills
Abstract
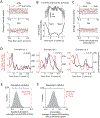
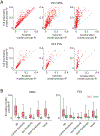
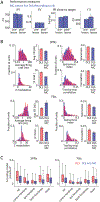
The basal ganglia are known to influence action selection and modulation of movement vigor, but whether and how they contribute to specifying the kinematics of learned motor skills is not understood. Here, we probe this question by recording and manipulating basal ganglia activity in rats trained to generate complex task-specific movement patterns with rich kinematic structure. We find that the sensorimotor arm of the basal ganglia circuit is crucial for generating the detailed movement patterns underlying the acquired motor skills. Furthermore, the neural representations in the striatum, and the control function they subserve, do not depend on inputs from the motor cortex. Taken together, these results extend our understanding of the basal ganglia by showing that they can specify and control the fine-grained details of learned motor skills through their interactions with lower-level motor circuits.
© 2021. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing Interests
The authors declare no competing interests.
Figures







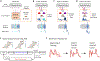






References
-
- Krakauer JW, Hadjiosif AM, Xu J, Wong AL & Haith AM Motor Learning. Compr. Physiol 9, 613–663 (2019). - PubMed
-
- Stephenson-Jones M, Samuelsson E, Ericsson J, Robertson B & Grillner S Evolutionary conservation of the basal ganglia as a common vertebrate mechanism for action selection. Curr. Biol. CB 21, 1081–1091 (2011). - PubMed
-
- Dudman JT & Krakauer JW The basal ganglia: from motor commands to the control of vigor. Curr. Opin. Neurobiol 37, 158–166 (2016). - PubMed
-
- Barnes TD, Kubota Y, Hu D, Jin DZ & Graybiel AM Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature 437, 1158–1161 (2005). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

