Complete Peripheral Blast Clearance is Superior to the Conventional Cut-Off of 1000/µL in Predicting Relapse in Pediatric Pre-B Acute Lymphoblastic Leukemia
- PMID: 34267453
- PMCID: PMC8239084
- DOI: 10.1007/s12288-020-01354-0
Complete Peripheral Blast Clearance is Superior to the Conventional Cut-Off of 1000/µL in Predicting Relapse in Pediatric Pre-B Acute Lymphoblastic Leukemia
Abstract
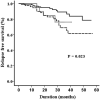
Risk-stratification has contributed to a dramatic improvement in survival in pediatric acute lymphoblastic leukemia (ALL). This study evaluated the utility of prephase response and day 15 bone marrow when a minimal residual disease (MRD) assessment was available. A file review of children aged ≤ 15 years diagnosed with precursor-B ALL from 2014 to 2019 was performed. The protocol used for risk stratification and treatment was based on a UKALL-2003 backbone. All patients received one week of prephase therapy comprised of intravenous dexamethasone in the first 48 h followed by oral prednisolone. The median age of the 255 patients in the study was 5 years. Following the prephase, the peripheral blood absolute blast count was 0 and ≥ 1000/µL blasts in 141 (56%) and 29 (11%), respectively. Ten of 199 (5%) patients with an evaluable day 15 bone marrow had M3 status. At the end of induction, 30 (12%), 127 (50%) and 98 (38%) patients belonged to the standard-risk, intermediate-risk and high-risk (HR) groups, respectively. An M3 day15 bone marrow was the sole reason for escalation in three (3%) of the patients in the HR group. A lack of complete clearance of peripheral blood blasts post-prephase [HR: 2.45 (1.04-5.75), p = 0.040] and a positive MRD [HR: 3.00 (1.28-7.02), p = 0.011] independently predicted risk of relapse. Complete blast clearance is superior to the traditional cut-off of 1000/µL in predicting relapse. The role of a day 15 bone marrow morphology is diminished when an end of induction MRD is available.
Keywords: Childhood leukemia; Early bone marrow; Poor prednisolone response; Prognostic marker; Rapid early response.
© Indian Society of Hematology and Blood Transfusion 2020.
Conflict of interest statement
Conflict of interestNone of the authors have anything to disclose.
Figures

References
-
- Athale UH, Gibson PJ, Bradley NM, Malkin DM, Hitzler J, POGO MRD Working Group (2016) Minimal residual disease and childhood leukemia: standard of care recommendations from the pediatric oncology group of ontario MRD working group. Pediatr Blood Cancer 63(6):973–82 - PubMed
-
- Vora A, Goulden N, Wade R, Mitchell C, Hancock J, Hough R, et al. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2013;14(3):199–209. doi: 10.1016/S1470-2045(12)70600-9. - DOI - PubMed
-
- Vora A, Goulden N, Mitchell C, Hancock J, Hough R, Rowntree C, et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2014;15(8):809–818. doi: 10.1016/S1470-2045(14)70243-8. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
