Microbial Communities and Interactions of Nitrogen Oxides With Methanogenesis in Diverse Peatlands of the Amazon Basin
- PMID: 34267733
- PMCID: PMC8276178
- DOI: 10.3389/fmicb.2021.659079
Microbial Communities and Interactions of Nitrogen Oxides With Methanogenesis in Diverse Peatlands of the Amazon Basin
Abstract
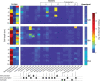
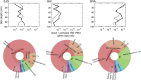
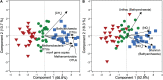
Tropical peatlands are hotspots of methane (CH4) production but present high variation and emission uncertainties in the Amazon region. This is because the controlling factors of methane production in tropical peats are not yet well documented. Although inhibitory effects of nitrogen oxides (NO x ) on methanogenic activity are known from pure culture studies, the role of NO x in the methane cycling of peatlands remains unexplored. Here, we investigated the CH4 content, soil geochemistry and microbial communities along 1-m-soil profiles and assessed the effects of soil NO x and nitrous oxide (N2O) on methanogenic abundance and activity in three peatlands of the Pastaza-Marañón foreland basin. The peatlands were distinct in pH, DOC, nitrate pore water concentrations, C/N ratios of shallow soils, redox potential, and 13C enrichment in dissolved inorganic carbon and CH4 pools, which are primarily contingent on H2-dependent methanogenesis. Molecular 16S rRNA and mcrA gene data revealed diverse and novel methanogens varying across sites. Importantly, we also observed a strong stratification in relative abundances of microbial groups involved in NO x cycling, along with a concordant stratification of methanogens. The higher relative abundance of ammonia-oxidizing archaea (Thaumarchaeota) in acidic oligotrophic peat than ammonia-oxidizing bacteria (Nitrospira) is noteworthy as putative sources of NO x . Experiments testing the interaction of NO x species and methanogenesis found that the latter showed differential sensitivity to nitrite (up to 85% reduction) and N2O (complete inhibition), which would act as an unaccounted CH4 control in these ecosystems. Overall, we present evidence of diverse peatlands likely differently affected by inhibitory effects of nitrogen species on methanogens as another contributor to variable CH4 fluxes.
Keywords: Amazon peatlands; methanogens; microbial communities and interactions; nitrogen oxides; peat geochemistry.
Copyright © 2021 Buessecker, Zamora, Sarno, Finn, Hoyt, van Haren, Urquiza Muñoz and Cadillo-Quiroz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Bai R., Wang J.-T., Deng Y., He J.-Z., Feng K., Zhang L.-M. (2017). Microbial community and functional structure significantly varied among distinct types of paddy soils but responded differently along gradients of soil depth layers. Front. Microbiol. 8:945. 10.3389/fmicb.2017.00945 - DOI - PMC - PubMed
-
- Barron A. R., Wurzburger N., Bellenger J. P., Wright S. J., Kraepiel A. M. L., Hedin L. O. (2008). Molybdenum limitation of asymbiotic nitrogen fixation in tropical forest soils. Nat. Geosci. 2 42–45. 10.1038/ngeo366 - DOI
LinkOut - more resources
Full Text Sources

