Hepatoselective Dihydroquinolizinone Bis-acids for HBsAg mRNA Degradation
- PMID: 34267883
- PMCID: PMC8274061
- DOI: 10.1021/acsmedchemlett.1c00228
Hepatoselective Dihydroquinolizinone Bis-acids for HBsAg mRNA Degradation
Abstract
Chronic hepatitis B (CHB) is characterized by high levels of hepatitis B virus (HBV) surface antigen (HBsAg) in blood circulation. A major goal of CHB interventions is reducing or eliminating this antigenemia; however, there are currently no approved methods that can do this. A novel family of compounds with a dihydroquinolizinone (DHQ) scaffold has been shown to reduce circulating levels of HBsAg in animals, representing a first for a small molecule. Reductions of HBsAg were a result of the compound's effect on HBsAg mRNA levels. However, commercial development by Roche of a DHQ lead compound, RG-7834, was stopped due to undisclosed toxicity issues. Herein we report our effort to convert the systemic RG7834 compound to a hepatoselective DHQ analog to limit its distribution to the bloodstream and thus to other body tissues.
© 2021 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
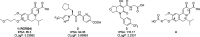
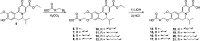



References
-
- Razavi-Shearer D.; Gamkrelidze I.; Nguyen M. H; Chen D.-S.; Van Damme P.; Abbas Z.; Abdulla M.; Abou Rached A.; Adda D.; Aho I.; et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 2018, 3 (6), 383–403. 10.1016/S2468-1253(18)30056-6. - DOI - PubMed
-
- Revill P. A.; Chisari F. V.; Block J. M.; Dandri M.; Gehring A. J.; Guo H.; Hu J.; Kramvis A.; Lampertico P.; Janssen H. L. A.; et al. A global scientific strategy to cure hepatitis B. Lancet Gastroenterol Hepatol 2019, 4 (7), 545–558. 10.1016/S2468-1253(19)30119-0. - DOI - PMC - PubMed
-
Erratum in:
- A global scientific strategy to cure hepatitis B. Lancet Gastroenterol Hepatol 2019, 4, 7545 - 558.10.1016/S2468-1253(19)30119-0 - PMC - PubMed
LinkOut - more resources
Full Text Sources
Chemical Information
Miscellaneous

