PI3K Promotes Basal Cell Carcinoma Growth Through Kinase-Induced p21 Degradation
- PMID: 34268113
- PMCID: PMC8276170
- DOI: 10.3389/fonc.2021.668247
PI3K Promotes Basal Cell Carcinoma Growth Through Kinase-Induced p21 Degradation
Abstract
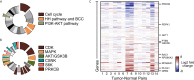
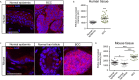
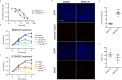
Basal cell carcinoma (BCC) is a locally invasive epithelial cancer that is primarily driven by the Hedgehog (HH) pathway. Advanced BCCs are a critical subset of BCCs that frequently acquire resistance to Smoothened (SMO) inhibitors and identifying pathways that bypass SMO could provide alternative treatments for patients with advanced or metastatic BCC. Here, we use a combination of RNA-sequencing analysis of advanced human BCC tumor-normal pairs and immunostaining of human and mouse BCC samples to identify a PI3K pathway expression signature in BCC. Pharmacological inhibition of PI3K activity in BCC cells significantly reduces cell proliferation and HH signaling. However, treatment of Ptch1fl/fl ; Gli1-CreERT2 mouse BCCs with the PI3K inhibitor BKM120 results in a reduction of tumor cell growth with no significant effect on HH signaling. Downstream PI3K components aPKC and Akt1 showed a reduction in active protein, whereas their substrate, cyclin-dependent kinase inhibitor p21, showed a concomitant increase in protein stability. Our results suggest that PI3K promotes BCC tumor growth by kinase-induced p21 degradation without altering HH signaling.
Keywords: PI3K - AKT pathway; atypical PKCι; basal cell carcinoma; hedgehog; p21.
Copyright © 2021 Chow, Jeon, Levee, Kaur, Cedeno, Doan and Atwood.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Raffel C, Jenkins RB, Frederick L, Hebrink D, Alderete B, Fults DW, et al. . Sporadic Medulloblastomas Contain PTCH Mutations. Cancer Res (1997) 47(5):842–5. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

