PPAR-α Agonist Fenofibrate Prevented Diabetic Nephropathy by Inhibiting M1 Macrophages via Improving Endothelial Cell Function in db/db Mice
- PMID: 34268320
- PMCID: PMC8275839
- DOI: 10.3389/fmed.2021.652558
PPAR-α Agonist Fenofibrate Prevented Diabetic Nephropathy by Inhibiting M1 Macrophages via Improving Endothelial Cell Function in db/db Mice
Abstract
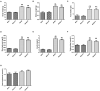
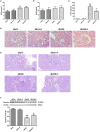
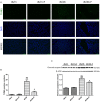
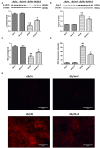
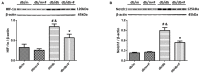
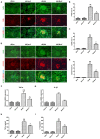
Background: Diabetic nephropathy (DN) is one of the major diabetic microvascular complications, and macrophage polarization plays a key role in the development of DN. Endothelial cells regulate macrophage polarization. Peroxisome proliferator-activated receptor (PPAR)-α agonists were demonstrated to prevent DN and improve endothelial function. In this study, we aimed to investigate whether PPAR-α agonists prevented DN through regulating macrophage phenotype via improving endothelial cell function. Methods: Eight-week-old male C57BLKS/J db/m and db/db mice were given fenofibrate or 1% sodium carboxyl methylcellulose by gavage for 12 weeks. Results: Db/db mice presented higher urinary albumin-to-creatinine ratio (UACR) than db/m mice, and fenofibrate decreased UACR in db/db mice. Fibrosis and collagen I were elevated in db/db mouse kidneys compared with db/m mouse kidneys; however, they were decreased after fenofibrate treatment in db/db mouse kidneys. Apoptosis and cleaved caspase-3 were enhanced in db/db mouse kidneys compared to db/m mouse kidneys, while fenofibrate decreased them in db/db mouse kidneys. Db/db mice had a suppression of p-endothelial nitric oxide synthase (eNOS)/t-eNOS and nitric oxide (NO), and an increase of angiopoietin-2 and reactive oxygen species (ROS) in kidneys compared with db/m mice, and fenofibrate increased p-eNOS/t-eNOS and NO, and decreased angiopoietin-2 and ROS in db/db mouse kidneys. Hypoxia-inducible factor (HIF)-1α and Notch1 were promoted in db/db mouse kidneys compared with db/m mouse kidneys, and were reduced after fenofibrate treatment in db/db mouse kidneys. Furthermore, the immunofluorescence staining indicated that M1 macrophage recruitment was enhanced in db/db mouse kidneys compared to db/m mouse kidneys, and this was accompanied by a significant increase of tumor necrosis factor (TNF)-α and interleukin (IL)-1β in kidneys and in serum of db/db mice compared with db/m mice. However, fenofibrate inhibited the renal M1 macrophage recruitment and cytokines associated with M1 macrophages in db/db mice. Conclusions: Our study indicated that M1 macrophage recruitment due to the upregulated HIF-1α/Notch1 pathway induced by endothelial cell dysfunction involved in type 2 diabetic mouse renal injury, and PPAR-α agonist fenofibrate prevented DN by reducing M1 macrophage recruitment via inhibiting HIF-1α/Notch1 pathway regulated by endothelial cell function in type 2 diabetic mouse kidneys.
Keywords: HIF-1α; Notch1; PPAR-α agonists; diabetic nephropathy; endothelial function; macrophages.
Copyright © 2021 Feng, Gao, Wang, Huang, Sun, Dong, Yu and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Nogueira GB, Punaro GR, Oliveira CS, Maciel FR, Fernandes TO, Lima DY, et al. . N-acetylcysteine protects against diabetic nephropathy through control of oxidative and nitrosative stress by recovery of nitric oxide in rats. Nitric Oxide - Biol Chem. (2018) 78:22–31. 10.1016/j.niox.2018.05.003 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

