This is a preprint.
Multi-site clinical validation of Isothermal Amplification based SARS-COV-2 detection assays using different sampling strategies
- PMID: 34268516
- PMCID: PMC8282105
- DOI: 10.1101/2021.07.01.21259879
Multi-site clinical validation of Isothermal Amplification based SARS-COV-2 detection assays using different sampling strategies
Update in
-
Multisite Clinical Validation of Isothermal Amplification-Based SARS-CoV-2 Detection Assays Using Different Sampling Strategies.Microbiol Spectr. 2021 Oct 31;9(2):e0084621. doi: 10.1128/Spectrum.00846-21. Epub 2021 Oct 20. Microbiol Spectr. 2021. PMID: 34668736 Free PMC article.
Abstract
Background: Isothermal amplification-based tests were developed as rapid, low-cost, and simple alternatives to real-time reverse transcriptase-polymerase chain reaction (RT-PCR) tests for SARS-COV-2 detection.
Methods: Clinical performance of two isothermal amplification-based tests (Atila Biosystems iAMP COVID-19 detection test and OptiGene COVID-19 Direct Plus RT-LAMP test) was compared to clinical RT-PCR assays using different sampling strategies. A total of 1378 participants were tested across four study sites.
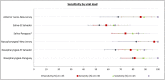
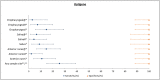
Results: Compared to standard of care RT-PCR testing, the overall sensitivity and specificity of the Atila iAMP test for detection of SARS-CoV-2 were 76.2% and 94.9%, respectively, and increased to 88.8% and 89.5%, respectively, after exclusion of an outlier study site. Sensitivity varied based on the anatomic collected site. Sensitivity for nasopharyngeal was 65.4% (range across study sites:52.8%-79.8%), mid-turbinate 88.2%, saliva 55.1% (range across study sites:42.9%-77.8%) and anterior nares 66.7% (range across study sites:63.6%-76.5%). The specificity for these anatomic collection sites ranged from 96.7% to 100%. Sensitivity improved in symptomatic patients (overall 82.7%) and those with a higher viral load (overall 92.4% for ct≤25). Sensitivity and specificity of the OptiGene Direct Plus RT-LAMP test, conducted at a single study-site, were 25.5% and 100%, respectively.
Conclusions: The Atila iAMP COVID test with mid-turbinate sampling is a rapid, low-cost assay for detecting SARS-COV-2, especially in symptomatic patients and those with a high viral load, and could be used to reduce the risk of SARS-COV-2 transmission in clinical settings. Variation of performance between study sites highlights the need for site-specific clinical validation of these assays before clinical adoption.
Conflict of interest statement
Conflict of Interest
The authors have nothing to declare. None of the companies had any role in design, analysis, interpretation, and finalization of the manuscript.
Figures



References
-
- WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data [Internet]. [cited 2021 May 11]. Available from: https://covid19.who.int/
-
- COVID-19 Vaccine Market Dashboard | UNICEF Supply Division [Internet]. [cited 2021 May 11]. Available from: https://www.unicef.org/supply/covid-19-vaccine-market-dashboard
-
- Cancino RS, Su Z, Mesa R, Tomlinson GE, Wang J. The impact of COVID-19 on cancer screening: challenges and opportunities [Internet]. Vol. 6, JMIR Cancer. JMIR Publications Inc.; 2020. [cited 2021 May 11]. Available from: https://pubmed.ncbi.nlm.nih.gov/33027039/ - PMC - PubMed
-
- In Vitro Diagnostics EUAs - Molecular Diagnostic Tests for SARS-CoV-2 | FDA [Internet]. [cited 2021 May 11]. Available from: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-em...
-
- Mitchell SL, St George K, Rhoads DD, Butler-Wu SM, Dharmarha V, McNult P, et al. Understanding, verifying, and implementing emergency use authorization molecular diagnostics for the detection of sars-cov-2 RNA [Internet]. Vol. 58, Journal of Clinical Microbiology. American Society for Microbiology; 2020. [cited 2021 May 11]. Available from: http://jcm.asm.org/ - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
