This is a preprint.
Short Telomeres and a T-Cell Shortfall in COVID-19: The Aging Effect
- PMID: 34268523
- PMCID: PMC8282112
- DOI: 10.1101/2021.05.19.21257474
Short Telomeres and a T-Cell Shortfall in COVID-19: The Aging Effect
Update in
-
Telomere-length dependent T-cell clonal expansion: A model linking ageing to COVID-19 T-cell lymphopenia and mortality.EBioMedicine. 2022 Apr;78:103978. doi: 10.1016/j.ebiom.2022.103978. Epub 2022 Apr 1. EBioMedicine. 2022. PMID: 35367774 Free PMC article.
Abstract
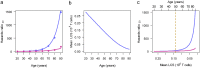
The slow pace of global vaccination and the rapid emergence of SARS-CoV-2 variants suggest recurrent waves of COVID-19 in coming years. Therefore, understanding why deaths from COVID-19 are highly concentrated among older adults is essential for global health. Severe COVID-19 T-cell lymphopenia is more common among older adults, and it entails poor prognosis. Much about the primary etiology of this form of lymphopenia remains unknown, but regardless of its causes, offsetting the decline in T-cell count during SARS-CoV-2 infection demands fast and massive T-cell clonal expansion, which is telomere length (TL)-dependent. We have built a model that captures the effect of age-dependent TL shortening in hematopoietic cells and its effect on T-cell clonal expansion capacity. The model shows that an individual with average hematopoietic cell TL (HCTL) at age twenty years maintains maximal T-cell clonal expansion capacity until the 6th decade of life when this capacity plummets by more than 90% over the next ten years. The collapse coincides with the steep increase in COVID-19 mortality with age. HCTL metrics may thus explain the vulnerability of older adults to COVID-19. That said, the wide inter-individual variation in HCTL across the general population means that some younger adults with inherently short HCTL might be at risk of severe COVID-19 lymphopenia and mortality from the disease.
Significance statement: Declining immunity with advancing age is a general explanation for the increased mortality from COVID-19 among older adults. This mortality far exceeds that from viral illnesses such as the seasonal influenza, and it thus requires specific explanations. One of these might be diminished ability with age to offset the development of severe T-cell lymphopenia (a low T-cell count in the blood) that often complicates COVID-19. We constructed a model showing that age-dependent shortening of telomeres might constrain the ability of T-cells of some older COVID-19 patients to undertake the massive proliferation required to clear the virus that causes the infection. The model predicts that individuals with short telomeres, principally seniors, might be at a higher risk of death from COVID-19.
Conflict of interest statement
Figures



References
-
- McClain M.T., Park L.P., Nicholson B, Veldman T, Zaas AK, Turner R, Lambkin-Williams R, Gilbert AS, Ginsburg GS, Woods CW. Longitudinal analysis of leukocyte differentials in peripheral blood of patients with acute respiratory viral infections. J Clin Virol. 58, 689–95 (2013). - PubMed
-
- Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, Belanger S, Abbott RK, Kim C, Choi J, Kato Y, Crotty EG, Kim C, Rawlings SA, Mateus J, Tse LPV, Frazier A, Baric R, Peters B, Greenbaum J, Ollmann Saphire E, Smith DM, Sette A, Crotty S. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell. 2020. Nov 12;183(4):996–1012.e19. doi: 10.1016/j.cell.2020.09.038. Epub 2020 Sep 16. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
