Clinical implementation of coronary computed tomography angiography for routine detection of cardiac allograft vasculopathy in heart transplant patients
- PMID: 34268796
- PMCID: PMC8519137
- DOI: 10.1111/tri.13973
Clinical implementation of coronary computed tomography angiography for routine detection of cardiac allograft vasculopathy in heart transplant patients
Abstract
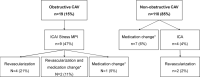
Cardiac allograft vasculopathy (CAV) is an accelerated form of coronary artery disease that affects long-term outcomes in heart transplant (HTx) patients. We prospectively evaluated the feasibility of coronary computed tomography angiography (CCTA) for the detection of CAV during clinical implementation at our center. All consecutive HTx patients >4 years post-transplant were actively converted from myocardial perfusion imaging to CCTA for the annual assessment of CAV. Between February 2018 and May 2019, 129/172 (75%) HTx patients underwent a CCTA. Renal impairment (n = 21/43) was the most frequent reason for patients could not undergo CCTA. CCTA image quality was good-excellent in 118/129 (92%) patients, and the radiation dose was 2.1 (1.6-2.8) mSv. CCTA showed obstructive CAV in 19/129 (15%) patients. Thirteen (10%) patients underwent additional tests, of which 8 patients underwent coronary revascularization within 90 days of CCTA. After 1 year, 3 additional coronary angiograms were performed, resulting in one revascularization in a patient with known severe CAV who developed ventricular tachycardia. One myocardial infarction after coronary stenting and 2 non-cardiac deaths were observed. CCTA can be successfully implemented for routine detection of CAV with good image quality and low radiation dose. CCTA allows CAV evaluation with the limited need for additional invasive testing.
Keywords: cardiac allograft vasculopathy; computed tomography angiography; coronary artery disease; heart transplantation; invasive coronary angiography.
© 2021 The Authors. Transplant International published by John Wiley & Sons Ltd on behalf of Steunstichting ESOT.
Conflict of interest statement
Dr. Budde reports institutional research support to the Erasmus MC from Siemens Healthineers outside he submitted work. Dr. Nieman reports unrestricted institutional grants from Siemens Healthineers, Bayer, and HeartFlow Inc., outside the submitted work. All other authors have not to disclose.
Figures


References
-
- Lund LH, Edwards LB, Kucheryavaya AY, et al. The registry of the international society for heart and lung transplantation: Thirty‐second official adult heart transplantation report–2015; focus theme: early graft failure. J Heart Lung Transplant 2015; 34: 1244. - PubMed
-
- Mehra MR, Crespo‐Leiro MG, Dipchand A, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy‐2010. J Heart Lung Transplant 2010; 29: 717. - PubMed
-
- Costanzo MR, Dipchand A, Starling R, et al. The international society of heart and lung transplantation guidelines for the care of heart transplant recipients. J Heart Lung Transplant 2010; 29: 914. - PubMed
-
- Wever‐Pinzon O, Romero J, Kelesidis I, et al. Coronary computed tomography angiography for the detection of cardiac allograft vasculopathy: a meta‐analysis of prospective trials. J Am Coll Cardiol 2014; 63: 1992. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

