Diarylethene-Based Photoswitchable Inhibitors of Serine Proteases
- PMID: 34268844
- PMCID: PMC8519022
- DOI: 10.1002/anie.202108847
Diarylethene-Based Photoswitchable Inhibitors of Serine Proteases
Abstract
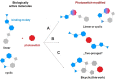
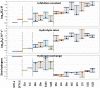
A bicyclic peptide scaffold was chemically adapted to generate diarylethene-based photoswitchable inhibitors of serine protease Bos taurus trypsin 1 (T1). Starting from a prototype molecule-sunflower trypsin inhibitor-1 (SFTI-1)-we obtained light-controllable inhibitors of T1 with Ki in the low nanomolar range, whose activity could be modulated over 20-fold by irradiation. The inhibitory potency as well as resistance to proteolytic degradation were systematically studied on a series of 17 SFTI-1 analogues. The hydrogen bond network that stabilizes the structure of inhibitors and possibly the enzyme-inhibitor binding dynamics were affected by isomerization of the photoswitch. The feasibility of manipulating enzyme activity in time and space was demonstrated by controlled digestion of gelatin-based hydrogel and an antimicrobial peptide BP100-RW. Finally, our design principles of diarylethene photoswitches are shown to apply also for the development of other serine protease inhibitors.
Keywords: bicyclic peptide; diarylethene photoswitch; hydrogel photoregulation; serine protease inhibitors; trypsin.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

