SphK1-driven autophagy potentiates focal adhesion paxillin-mediated metastasis in colorectal cancer
- PMID: 34268882
- PMCID: PMC8419751
- DOI: 10.1002/cam4.4129
SphK1-driven autophagy potentiates focal adhesion paxillin-mediated metastasis in colorectal cancer
Abstract
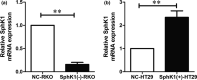
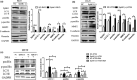
Invasion and metastasis are the main causes of colorectal cancer (CRC)-related death. Accumulating evidence suggested that sphingosine kinase 1 (SphK1) promoted the metastasis of CRC and autophagy played an important role in SphK1 promoting the metastasis of malignancy. However, the mechanism by which SphK1-driven autophagy promotes invasion and metastasis in CRC remains to be clarified. In the present study, immunohistochemical detection showed the expression of SphK1 and paxillin was higher in human CRC tissues than those of normal colorectal mucosal tissues, they were both associated with TNM staging, lymphatic, and distance metastasis. In addition, study of in situ tumor transplantation model in nude mice showed that the suppression of SphK1 inhibited the growth of colonic orthotopic implantation tumors and the expression of paxillin, p-paxillin, LC3 in the tumor. So, SphK1 may promote CRC metastasis via inducing the expression of paxillin expression and its phosphorylation, in vivo. Furthermore, results of CCK8 assay, transwell and wound healing assays showed that SphK1 promoted the viability, invasion, and metastasis of CRC cells. Transmission electron microscopy detection showed that SphK1 is the key factor in autophagy induction in CRC cells. Moreover, western blot examination indicated that the expression of LC3Ⅱ/Ⅰ, paxillin, p-paxillin, MMP-2, and vimentin was enhanced in SphK1-overexpressed CRC cells and suppressed in SphK1 knockdown CRC cells, meanwhile, the expression of E-cadherin was suppressed in SphK1-overexpressed CRC cells and enhanced in SphK1 knockdown CRC cells. Suppression of autophagy by 3MA reversed the expression of paxillin and its phosphorylation in SphK1-overexpressed CRC cells, indicated that SphK1-driven autophagy induced the expression of paxillin and its phosphorylation in CRC cells. Together, these findings reveal that SphK1-driven autophagy may promote the invasion and metastasis of CRC via promoting the expression of focal adhesion paxillin and its phosphorylation.
Keywords: autophagy; colorectal cancer; metastases; paxillin; sphingosine kinase 1.
© 2021 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. - PubMed
-
- Pyne NJ, El Buri A, Adams DR, Pyne S. Sphingosine 1‐phosphate and cancer. Adv Biol Regul. 2018;68:97‐106. - PubMed
-
- Wang D, Bao F, Teng Y, Li Q, Li J. MicroRNA‐506‐3p initiates mesenchymal‐to‐epithelial transition and suppresses autophagy in osteosarcoma cells by directly targeting SPHK1. Biosci Biotechnol Biochem. 2019;83(5):836‐844. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2017GXNSFAA198019/Natural Science Foundation of Guangxi Province
- 2020GXNSFAA159056/Natural Science Foundation of Guangxi Province
- GJPY2018010/Natural Science Foundation Fostering Science Foundation of the Second Affiliated Hospital of Guangxi Medical University
- 81460380/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Miscellaneous

