Clinical evaluation of large volume subcutaneous injection tissue effects, pain, and acceptability in healthy adults
- PMID: 34268888
- PMCID: PMC8742644
- DOI: 10.1111/cts.13109
Clinical evaluation of large volume subcutaneous injection tissue effects, pain, and acceptability in healthy adults
Abstract
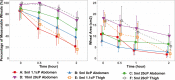
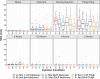
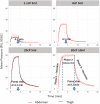
Determining feasibility and tolerability of large volume viscous subcutaneous injection may enable optimized, intuitive delivery system design. A translational early feasibility clinical study examined large volume subcutaneous injection viability, tolerability, acceptability, tissue effects and depot location for ~1, 8, and 20 cP injections at volumes up to 10 ml in the abdomen and 5 ml in the thigh in 32 healthy adult subjects. A commercial syringe pump system delivered 192 randomized, constant rate (20 µl/s) injections (6/subject) with in-line injection pressure captured versus time. Deposition location was qualified via ultrasound. Tissue effects and pain tolerability were monitored through 2 hours post-injection with corresponding Likert acceptability questionnaires administered through 72 hours. All injection conditions were feasible and well-tolerated with ≥79.3% favorable subject responses for injection site appearance and sensation immediately post-injection, increasing to ≥96.8% at 24 hours. Mean subject pain measured via 100 mm visual analog scale increased at needle insertion (6.9 mm, SD 10.8), peaked during injection (26.9 mm, SD 21.7) and diminished within 10 minutes post-removal (1.9 mm, SD 4.2). Immediate injection site wheal (90.9%) and erythema (92.6%) formation was observed with progressive although incomplete resolution through 2 hours (44.6% and 11.4% remaining, respectively). Wheal resolution occurred more rapidly at lower viscosities. Most subjects (64.5%) had no preference between abdomen and thigh. Correlations between tissue effects, injection pressure and pain were weak (Pearson's rho ± 0-0.4). The large volume injections tested, 1-20 cP viscosities up to 10 ml in the abdomen and 5 ml in the thigh, are feasible with good subject acceptability and rapid resolution of tissue effects and pain.
© 2021 Becton, Dickinson and Company. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
W.D.W., D.R.M., D.E.S., R.J.P., and N.G.B. were employees of the study sponsor and funding entity, BD (Becton, Dickinson and Company), when the study was performed and may also be stockholders.
Figures

References
-
- Pivot X, Gligorov J, Müller V, et al. Patients’ preferences for subcutaneous trastuzumab versus conventional intravenous infusion for the adjuvant treatment of HER2‐positive early breast cancer: final analysis of 488 patients in the international, randomized, two‐cohort PrefHer study. Ann Oncol. 2014;25:1979‐1987. - PubMed
-
- Rule S, Collins GP, Samanta K. Subcutaneous vs intravenous rituximab in patients with non‐Hodgkin lymphoma: a time and motion study in the United Kingdom. J Med Econ. 2014;17:459‐468. - PubMed
-
- Shapiro R. Subcutaneous immunoglobulin therapy by rapid push is preferred to infusion by pump: a retrospective analysis. J Clin Immunol. 2010;30:301‐307. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

