Koi sleepy disease as a pathophysiological and immunological consequence of a branchial infection of common carp with carp edema virus
- PMID: 34269137
- PMCID: PMC8288041
- DOI: 10.1080/21505594.2021.1948286
Koi sleepy disease as a pathophysiological and immunological consequence of a branchial infection of common carp with carp edema virus
Abstract
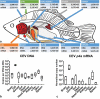
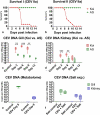
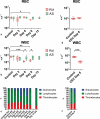
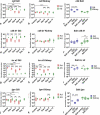
Gills of fish are involved in respiration, excretion and osmoregulation. Due to numerous interactions between these processes, branchial diseases have serious implications on fish health. Here, "koi sleepy disease" (KSD), caused by carp edema virus (CEV) infection was used to study physiological, immunological and metabolic consequences of a gill disease in fish. A metabolome analysis shows that the moderately hypoxic-tolerant carp can compensate the respiratory compromise related to this infection by various adaptations in their metabolism. Instead, the disease is accompanied by a massive disturbance of the osmotic balance with hyponatremia as low as 71.65 mmol L-1, and an accumulation of ammonia in circulatory blood causing a hyperammonemia as high as 1123.24 µmol L-1. At water conditions with increased ambient salt, the hydro-mineral balance and the ammonia excretion were restored. Importantly, both hyponatremia and hyperammonemia in KSD-affected carp can be linked to an immunosuppression leading to a four-fold drop in the number of white blood cells, and significant downregulation of cd4, tcr a2 and igm expression in gills, which can be evaded by increasing the ion concentration in water. This shows that the complex host-pathogen interactions within the gills can have immunosuppressive consequences, which have not previously been addressed in fish. Furthermore, it makes the CEV infection of carp a powerful model for studying interdependent pathological and immunological effects of a branchial disease in fish.
Keywords: Carp edema virus; gills; hyperammonemia; hyponatremia; immunosuppression; koi sleepy disease; metabolome; osmoregulation.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures







References
-
- Lewisch E, Gorgoglione B, Way K, et al. Carp edema virus/Koi sleepy disease: an emerging disease in Central-East Europe. Transbound Emerg Dis. 2015;62:6–12. - PubMed
-
- Way K, Haenen O, Stone D, et al. Emergence of carp edema virus (CEV) and its significance to European common carp and koi Cyprinus carpio. Dis Aquat Organ. 2017;126:155–166. - PubMed
-
- Adamek M, Oschilewski A, Wohlsein P, et al. Experimental infections of different carp strains with the carp edema virus (CEV) give insights into the infection biology of the virus and indicate possible solutions to problems caused by koi sleepy disease (KSD) in carp aquaculture. Vet Res. 2017;48:12. - PMC - PubMed
-
- Miyazaki T, Isshiki T, Katsuyuki H.. Histopathological and electron microscopy studies on sleepy disease of koi Cyprinus carpio koi in Japan. Dis Aquat Organ. 2005;65:197–207. - PubMed
-
- Powell MD, Reynolds P, Kristensen T. Freshwater treatment of amoebic gill disease and sea-lice in seawater salmon production: considerations of water chemistry and fish welfare in Norway. Aquaculture. 2015;448:18–28.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
