SYNGR4 and PLEKHB1 deregulation in motor neurons of amyotrophic lateral sclerosis models: potential contributions to pathobiology
- PMID: 34269186
- PMCID: PMC8463983
- DOI: 10.4103/1673-5374.317960
SYNGR4 and PLEKHB1 deregulation in motor neurons of amyotrophic lateral sclerosis models: potential contributions to pathobiology
Abstract
Amyotrophic lateral sclerosis is the most common adult-onset neurodegenerative disease affecting motor neurons. Its defining feature is progressive loss of motor neuron function in the cortex, brainstem, and spinal cord, leading to paralysis and death. Despite major advances in identifying genes that can cause disease when mutated and model the disease in animals and cellular models, it still remains unclear why motor symptoms suddenly appear after a long pre-symptomatic phase of apparently normal function. One hypothesis is that age-related deregulation of specific proteins within key cell types, especially motor neurons themselves, initiates disease symptom appearance and may also drive progressive degeneration. Genome-wide in vivo cell-type-specific screening tools are enabling identification of candidates for such proteins. In this minireview, we first briefly discuss the methodology used in a recent study that applied a motor neuron-specific RNA-Seq screening approach to a standard model of TAR DNA-binding protein-43 (TDP-43)-driven amyotrophic lateral sclerosis. A key finding of this study is that synaptogyrin-4 and pleckstrin homology domain-containing family B member 1 are also deregulated at the protein level within motor neurons of two unrelated mouse models of mutant TDP-43 driven amyotrophic lateral sclerosis. Guided by what is known about molecular and cellular functions of these proteins and their orthologs, we outline here specific hypotheses for how changes in their levels might potentially alter cellular physiology of motor neurons and detrimentally affect motor neuron function. Where possible, we also discuss how this information could potentially be used in a translational context to develop new therapeutic strategies for this currently incurable, devastating disease.
Keywords: TAR DNA-binding protein-43; amyotrophic lateral sclerosis; glucagon-like peptide-1 receptor; motor neuron disease; mouse model; neurodegeneration; phosphatidylserine; pleckstrin homology domain; synaptogyrin; vesicle transport.
Conflict of interest statement
None
Figures
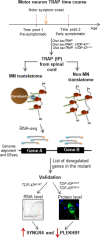
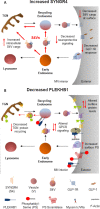
References
-
- Arnold ES, Ling SC, Huelga SC, Lagier-Tourenne C, Polymenidou M, Ditsworth D, Kordasiewicz HB, McAlonis-Downes M, Platoshyn O, Parone PA, Da Cruz S, Clutario KM, Swing D, Tessarollo L, Marsala M, Shaw CE, Yeo GW, Cleveland DW. ALS-linked TDP-43 mutations produce aberrant RNA splicing and adult-onset motor neuron disease without aggregation or loss of nuclear TDP-43. Proc Natl Acad Sci U S A. 2013;110:E736–745. - PMC - PubMed
-
- Belfort GM, Kandror KV. Cellugyrin and synaptogyrin facilitate targeting of synaptophysin to a ubiquitous synaptic vesicle-sized compartment in PC12 cells. J Biol Chem. 2003;278:47971–47978. - PubMed
-
- Brown RH, Al-Chalabi A. Amyotrophic lateral sclerosis. N Engl J Med. 2017;377:162–172. - PubMed
-
- Cook C, Petrucelli L. Genetic convergence brings clarity to the enigmatic red line in ALS. Neuron. 2019;101:1057–1069. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

